SulfurâIodine (SâI) cycle is one of the efficient and ecoâfriendly thermochemical methods for hydrogen production. SO
3 decomposition is the most endothermic step in the SâI cycle. The development of a catalytic system to achieve high activity and stability is a major challenge for the SO
3 decomposition due to high temperature and corrosive environment of the reaction. CuFe
2O
4 catalyst supported over βâSiC foam is synthesized using wet impregnation method followed by high temperature solid state route. The prepared catalyst is characterized using various techniques such as XRD, FTIR, BET, FESEM, TEM and HR-TEM. The synthesized catalyst is activity tested at atmospheric condition in the temperature range of 800â900 ËC and at 8.1 h
-1 WHSV.
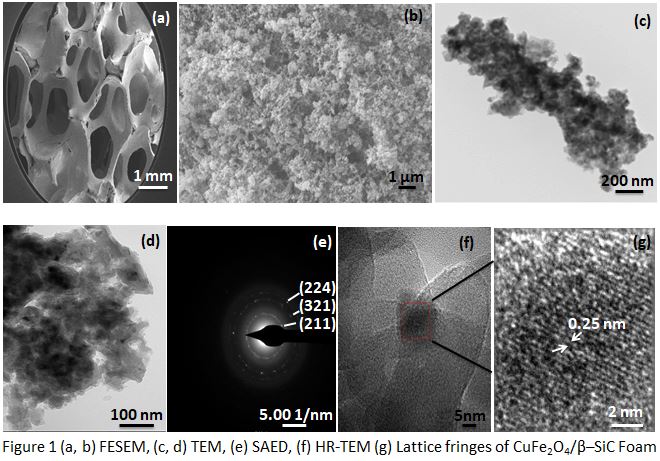
Figure 1 shows (a, b) FESEM, (c, d) TEM, (e) SAED, (f) HR-TEM, (g) Lattice fringes of CuFe2O4/βâSiC Foam. CuFe2O4 particles were well dispersed on the βâSiC Foam as shown in FESEM and TEM images. The concentric rings in the selected area electron diffraction (SAED) pattern showed the nanocrystalline nature of the CuFe2O4/βâSiC Foam. The rings indexed to the planes of tetragonal CuFe2O4 (PDFâ 34-0425). The magnified view of the selected portion of the HRâTEM image showed the lattice fringes of CuFe2O4/βâSiC Foam, the interplanar distance was found to be 0.25 nm corresponded to the (211) plane of tetragonal CuFe2O4. The activity of the catalyst approached equilibrium conversion with increase in temperature. Issues such as low catalytic activity, pressure drop and temperature gradient can be tackled with the synthesized catalyst because foam structure of a support is considered to have high heat transfer due to its interconnected structure and low pressure drop as compared to pellet form in a packed bed reactor.