While propylene has been traditionally produced with steam and fluid catalytic cracking, dehydrogenation of propane is an alternative. Industrially, Cr oxide catalysts on alumina are used. Herein, we present a study of the reaction on oxidized and reduced Cr
2O
3(0001). We used first-principles calculations to postulate the reaction mechanism. Plane-wave DFT with the Hubbard correction (DFT+U) was used to obtain the entire energetics and the corresponding potential energy surfaces. The catalyst was modelled as a 12-layer slab. We modelled every possible elementary reaction step for hydrogen abstraction and energetically most probable C-C breaking steps, thus accounting for coking and catalyst deactivation. Then, a kinetic Monte Carlo model was proposed and run on a lattice with four types of active sites (corresponding to Cr and O atoms in oxidized and reduced environment).
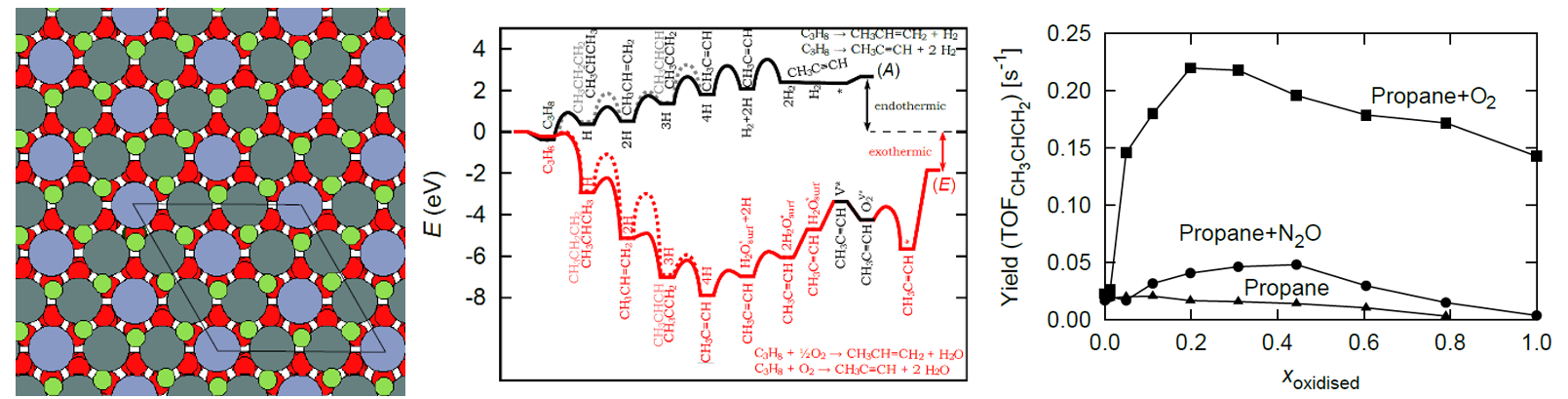
We show that the oxidized and reduced surface behave markedly different. On the reduced surface, the reaction is endothermic and the interaction of the intermediates with the surface is weaker, resulting in lower activity but high selectivity to propylene. On the oxidized surface, the activity increases but selectivity plummets as CO2 is mostly produced. The selectivity is strongly affected by the oxidant pressure and type (CO2, N2O, O2). Moreover, we show that there exists an optimum degree of surface oxidation (around 20 %), where propylene yields are highest.
The observed apparent activation barrier was 1.39 eV and 1.34 eV on the reduced and oxidized surface, respectively. Propylene and some propyne are produced according to this mechanism: C3H8 â CH3CHCH3 â CH3CHCH2 â CH3CCH2 â CH3CCH. Some C1 and C2 products are formed. At higher temperatures, the catalyst undergoes deactivation due to the formation of the CC* and C* species, which bind to the catalyst and eventually form coke.