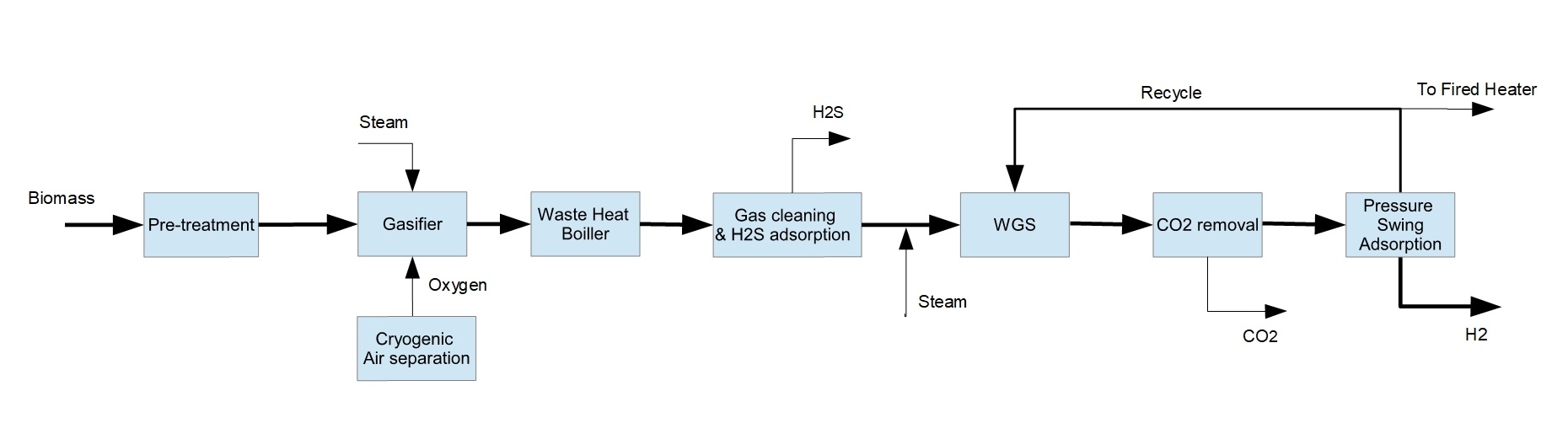
Hydrogen is an important energy vector which has immense potential to lower greenhouse gas (GHG) emissions in hard to abate industries. Currently H
2 is produced predominantly from fossil sources which entail associated CO
2 emissions. Biomass, on the other hand, is a natural sink for atmospheric CO
2 and if it is used for production of H
2 or other fuels, it would be almost carbon-neutral. If carbon capture is added to the process with permanent CO
2 storage, the final product would be carbon negative. This means that the process removes atmospheric CO
2. Here we evaluate a thermochemical pathway for production of H
2 from biomass (see Figure 1). It is comprised of an entrained flow gasifier (EFG), hot gas cleaning unit, water gas shift (WGS) reactor, CO
2 removal unit and pressure swing adsorption (PSA) unit. We have chosen technologies that are high in technology readiness level (TRL) and can be implemented today. The process is modeled in Aspen HYSYS
® for mass and energy balances while available literature data is used to estimate associated costs of the process. The results are benchmarked against the standard steam methane reforming (SMR) with respect to economics as well as GHG emissions. Through sensitivity analysis, key factors affecting economics and emissions are highlighted. This work helps to gain insight on the viability of sustainable H
2 production from biomass. Gasification of available biomass should be considered in addition to H
2 from natural gas or water electrolysis to supply the emerging hydrogen need in industry and economy.