2022 Annual Meeting
(497c) PLG Microparticles for Small Molecule Delivery to Muscle Cells
Authors
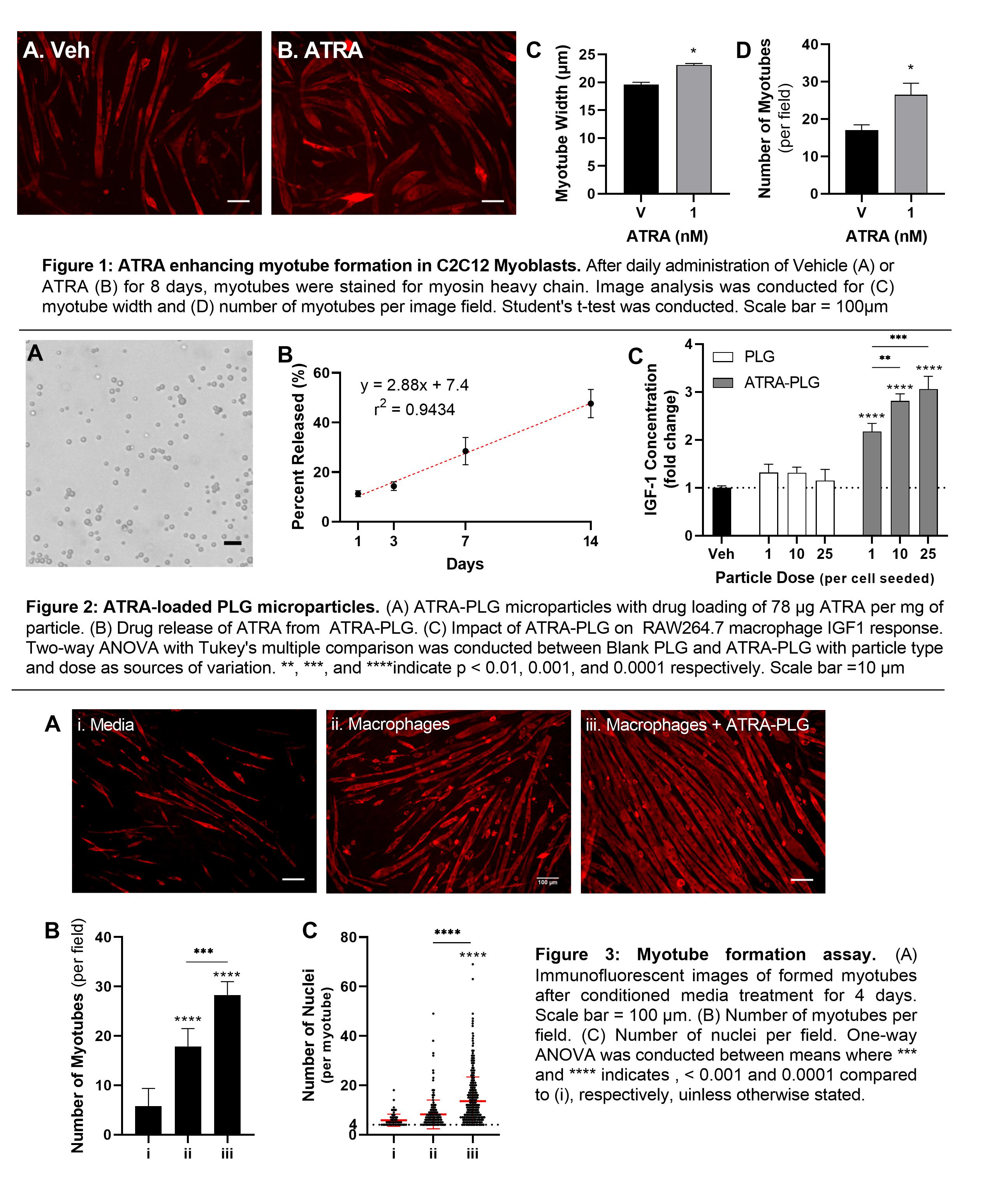
Methods: To confirm ATRAâs muscle-formative ability, ATRA was used to treat C2C12 myoblasts daily for 8 days. After performing immunofluorescence microscopy for myosin heavy chain, image analysis was used to quantify myotube formation and growth. ATRA-loaded PLG particles were synthesized using a single emulsion/solvent-evaporation technique by adding 5 mg/mL of ATRA to the organic phase for ATRA-PLG particles. Drug loading was determined using UV-vis spectroscopy. To confirm the bioactivity of ATRA after encapsulation, RAW264.7 macrophages were incubated with ATRA-PLG particles for 24 hours, then media were collected for IGF1 ELISA analysis. To investigate the impact of ATRA-PLG particles on macrophage-induced myoblast fusion into myotubes (an in vitro model of muscle regeneration), macrophages were treated with ATRA-PLG particles for 24 hours, after which macrophage conditioned media was collected and used to treat C2C12 myoblasts daily for 3 days. Control groups included conditioned media from macrophages without particle treatment and media conditioned with ATRA particles alone. After performing immunofluorescence microscopy for myosin heavy chain, image analysis was used to quantify myotube formation and growth.
Results and Discussion: In a myotube formation assay using C2C12 murine myoblasts, ATRA was able to successfully increase myotube formation as shown by myotube width and number of myotubes (Figure 1). ATRA was successfully incorporated into PLG microparticles, producing spherical and monodisperse particles with a diameter of 2.9 µm (Figure 2A). Drug loading for the ATRA-PLG particles were 78 µg of ATRA per milligram of particle with a linear drug release of 2.88% released per day (Figure 2B). When incubated with macrophages, ATRA particles increase IGF1 secretion into the media compared to blank PLG microparticles (Figure 2C), indicating that the ATRA remained bioactive after encapsulation. In the myotube formation assay, media collected from macrophages treated with ATRA-PLG particles induced myoblasts to fuse more readily into myotubes (Figure 3A) compared to all other treatments, as quantified by the number of myotubes per image field (Figure 3B) and number of incorporated nuclei per myotube (Figure 3C). The data indicate that while untreated macrophages and ATRA released from the particles increase myotube formation, there is an enhanced effect when macrophages are pre-treated with the ATRA-PLG particles. Since macrophages are present in skeletal muscle, we expect that there are opportunities to modulate muscle growth with ATRA-PLG particles indirectly through macrophages and directly through ATRA acting on muscle.
Conclusion: ATRA can be encapsulated into PLG microparticles while maintaining bioactivity. Treatment of macrophages with ATRA-PLG particles changes the secretome in a way that enhances myotube formation compared to media from untreated macrophages or media containing ATRA, but no macrophage-derived factors. The data suggest that elevated levels of IGF1 play a role, but changes in other myogenic or myostatic factors cannot be ruled out. Future studies will assess the efficacy of ATRA-PLG particle delivery on muscle growth in a mouse model of disuse muscle atrophy.
(1) Zhang, Y.; Pan, X.; Sun, Y.; Geng, Y. J.; Yu, X. Y.; Li, Y. The Molecular Mechanisms and Prevention Principles of Muscle Atrophy in Aging. 2018, 1088, 347â368. https://doi.org/10.1007/978-981-13-1435-3_16.
(2) Tidball, J. G.; Welc, S. S. Macrophage-Derived IGF-1 Is a Potent Coordinator of Myogenesis and Inflammation in Regenerating Muscle. 2015, 23 (7), 1134â1135. https://doi.org/10.1038/mt.2015.97.
(3) Széles, L.; Póliska, S.; Nagy, G.; Szatmari, I.; Szanto, A.; Pap, A.; Lindstedt, M.; Santegoets, S. J. A. M.; Rühl, R.; Dezsö, B.; Nagy, L. Research Resource: Transcriptome Profiling of Genes Regulated by RXR and Its Permissive and Nonpermissive Partners in Differentiating Monocyte-Derived Dendritic Cells. 2010, 24 (11), 2218â2231. https://doi.org/10.1210/me.2010-0215.
(4) Mezquita, B.; Mezquita, C. Two Opposing Faces of Retinoic Acid: Induction of Stemness or Induction of Differentiation Depending on Cell-Type. Biomolecules 2019, 9 (10). https://doi.org/10.3390/biom9100567.
(5) Ozpolat, B.; Lopez-Berestein, G.; Adamson, P.; Fu, C. J.; Williams, A. H. Pharmacokinetics. 2003, 10.
(6) Kawakami, S.; Opanasopit, P.; Yokoyama, M.; Chansri, N.; Yamamoto, T.; Okano, T.; Yamashita, F.; Hashida, M. Biodistribution Characteristics of All-Trans Retinoic Acid Incorporated in Liposomes and Polymeric Micelles Following Intravenous Administration. J. Pharm. Sci. 2005, 94 (12), 2606â2615. https://doi.org/10.1002/jps.20487.
(7) Szuts, E. Z.; Harosi, F. I. Solubility of Retinoids in Water. Arch Biochem Biophys 1991, 287 (2), 297â304. https://doi.org/10.1016/0003-9861(91)90482-x.
(8) Tolleson, W.; Cherng, S.-H.; Xia, Q.; Boudreau, M.; Yin, J.; Wamer, W.; Howard, P.; Yu, H.; Fu, P. Photodecomposition and Phototoxicity of Natural Retinoids. Int. J. Environ. Res. Public. Health 2005, 2 (1), 147â155. https://doi.org/10.3390/ijerph2005010147.
(9) David, M.; Hodak, E.; Lowe, N. J. Adverse Effects of Retinoids. Med. Toxicol. 1988, 3 (4), 273â288. https://doi.org/10.1007/BF03259940.