2022 Annual Meeting
(482f) Oxidation Rate and Leaching of Flame-Made Nanosilver By Reactive Molecular Dynamics
Authors
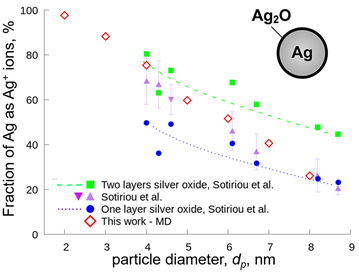
Here, the oxidation of silver nanoparticles made by flame spray pyrolysis is investigated at high temperature by reactive molecular dynamics. The oxidation rate equation is obtained ab initio as a function of temperature and particle coverage. Figure 1 shows the fraction of silver ions in nanosilver particles as function of particle size obtained by reactive molecular dynamics simulations (diamonds). The results are in excellent agreement with experiments of flame- and wet-made nanosilver (triangles) in aqueous suspensions. For comparison, the estimated silver ion fractions assuming one (circles) and two (squares) silver oxide layers are shown. Nanosilver particles with diameter of 3 â 4 nm exhibit a large fraction of silver ions, which are present in the form of silver oxide at the particle surface. This fraction corresponds to a double layer of silver oxide. Larger silver nanoparticles, having diameter of 7 â 8 nm, exhibit a smaller fraction of silver ions that corresponds to a single silver oxide layer, consistent with experiments (Sotiriou et al., 2012). In addition, the effect of particle size on the leaching rate of nanosilver in aqueous solutions is quantified to understand the mechanism of silver ion release, which is responsible for the nanosilver toxic manifestations. Minimising these release rates can enable the expanded use of silver nanoparticles and their nanocomposites as bioimaging materials or plasmonic biosensors.
References
Sotiriou, G. A., Meyer, A., Knijnenburg, J. T., Panke, S., & Pratsinis, S. E. (2012). Quantifying the origin of released Ag+ ions from nanosilver. Langmuir, 28(45), 15929-15936.