In
2O
3 supported on ZrO
2 is a promising catalyst for hydrogenation of carbon dioxide to methanol, as it has high stability and selectivity to methanol. This study aims to investigate the methanol and CO formation pathways on the more stable In
2O
3(111) phase and to develop an accurate microkinetic model. DFT simulations were performed with c-In
2O
3(111) supported over m-ZrO
2(111). Supporting In
2O
3 over ZrO
2 favored oxygen vacancy formation by 0.42 eV compared to the non-supported catalyst, facilitating the formation of the active sites. CO
2* underwent hydrogenation to form bidentate HCOO*, followed by H
2COO*, occupying the oxygen vacancy site. Among the different possible routes, the H
2CO-O cleavage route was found to be more favorable. The C-O cleavage in H
2COO* was the most energetically unfavorable step of the methanol formation pathway, proceeding either to give H
2CO* (activation barrier of 1.01 eV) or to give the H
3CO* species (activation barrier of 1.14 eV). In the parallel reverse water gas shift (RWGS) reaction route, CO
2* is hydrogenated to give COOH*, followed by the energetically unfavorable C-O cleavage to give CO* (activation barrier of 1.01 eV). Microkinetic modeling was done by calculating the required kinetic parameters from the DFT results using transition-state theory. The selectivity of methanol was more than 90% for temperatures ranging from 473-573 K at 50 bar pressure, which agrees with previously reported experimental results from the literature. This is attributed to HCOO* formation having a lower activation barrier than that of COOH*. The CO
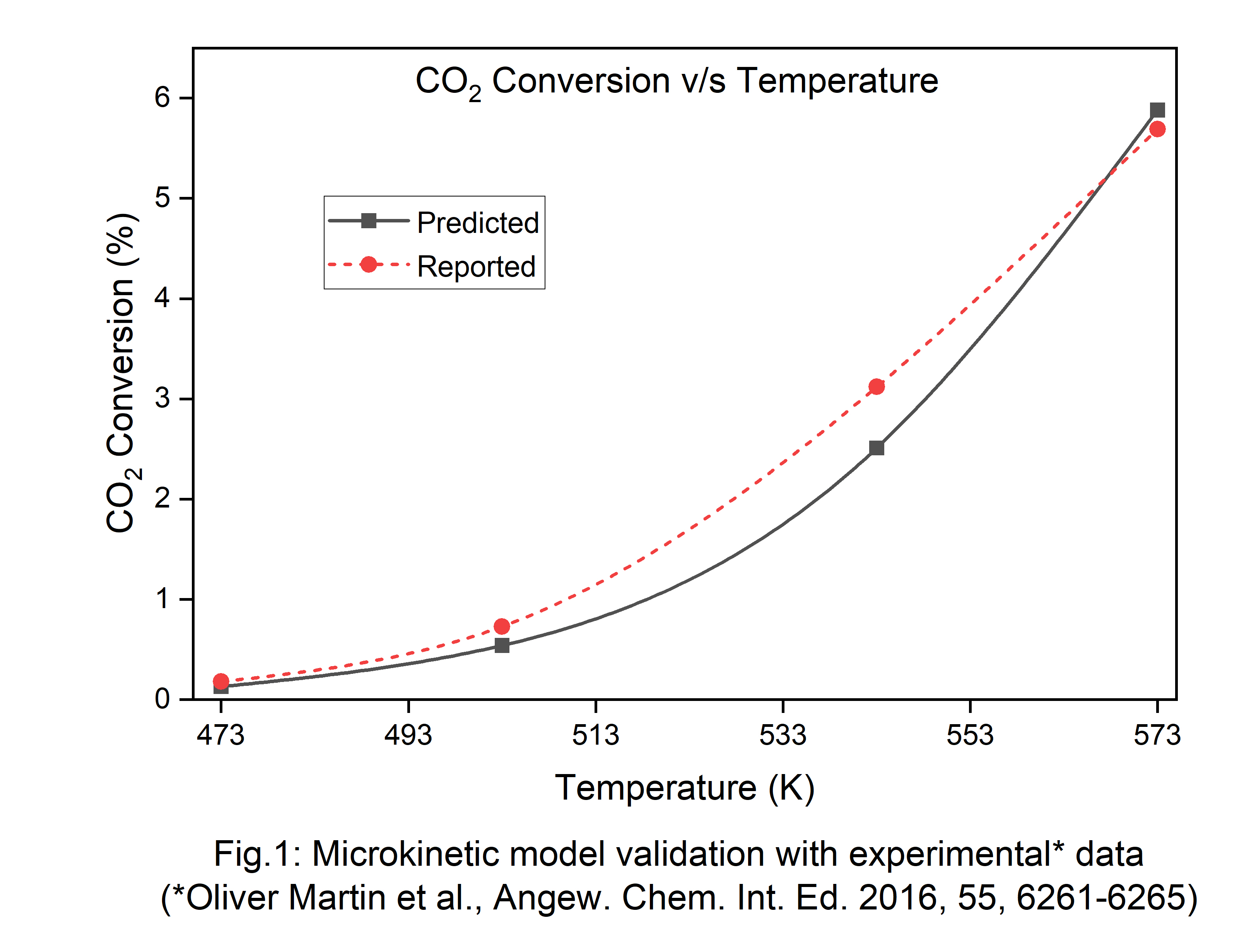
2 conversion values also agree with the reported catalyst performance data (Fig.1). Under reaction conditions, the catalyst surface was found to be hydroxylated, hindering further heterolytic dissociation of H
2. Sensitivity analysis revealed that H
2 dissociation is the rate-determining step for methanol formation, supporting this observation. Improving H
2 dissociative adsorption capacity of the catalyst is a possible strategy to enhance methanol yield.