Valorizing biomass-derived lactones such as mevalonolactone (MVL), triacetic acid lactone (TAL), and γ-valerolactone (GVL) involve removal of oxygen-based functionalities to get a variety of high-value chemicals and liquid fuels. Reactions like dehydration, ring-opening, and decarboxylation reactions are often used to accomplish these conversions. Under specific catalytic and solvent conditions, these reactions may exhibit marked differences in reaction kinetics and selectivity. For example, under aqueous conditions, the ring-opening and decarboxylation reactions of TAL have been shown to occur at low temperatures with higher conversion and selectivity, demonstrating solvent's effect. Free energy calculations via ab-initio molecular dynamics (MD) simulations have also been performed to investigate the solvent's effect on saturated pyrones' ring-opening and decarboxylation reactions. Here, a similar study is conducted to elucidate the effect of polar (protic and aprotic) and apolar solvent media on the acid-catalyzed dehydration reaction of biomass-derived lactones. In further combination with classical MD simulations of bulk and mixed solvent environments, the solvent's spatial arrangement in the vicinity of reactant is studied.
Classical MD simulations are performed with GROMACS software package. Water and THF are used for polar and cyclohexane for solvent media. While the water is modeled using SPC/E potential, the reactant, 4-hydroxy-6-methyllactone (HML), THF, and cyclohexane are modeled using OPLS all-atom potential. The dehydration reaction of HML in the two solvent media is studied using Car-Parrinello Molecular Dynamics (CPMD) simulations. Ultrasoft psuedopotentials with BLYP functionals are used to treat the core and valence electrons, respectively. After initial wavefunction and geometry optimization, the simulation system are equilibrated for 50 ps. Subsequently, free energy calculations are performed in 2d collective variable space using metadynamics.
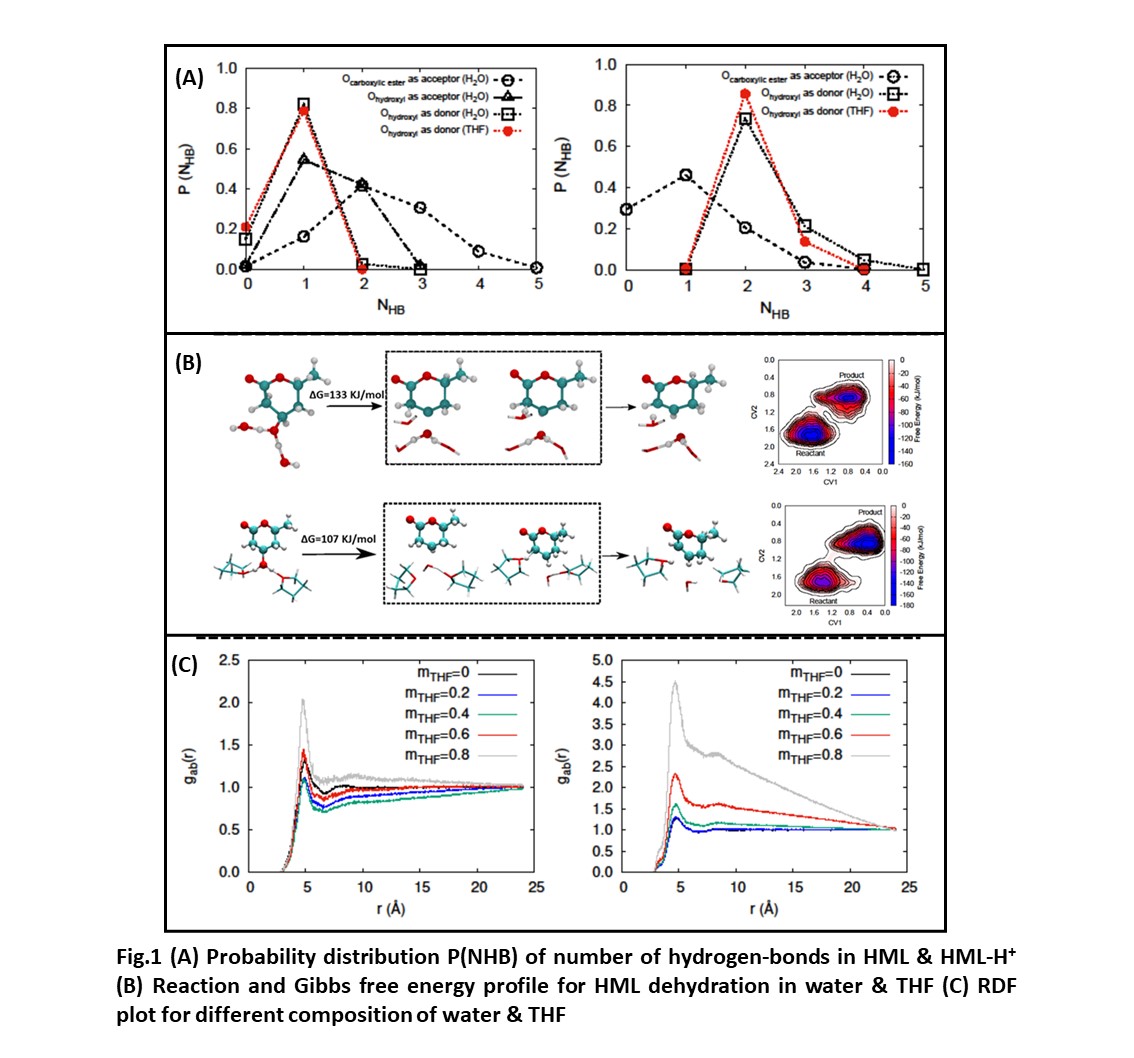
From both classical MD and CPMD, HML is observed to form 3 to 5 hydrogen bonds in water and 1 to 2 hydrogen bonds in THF (Fig.1(A)). For HML-H+ complex in CPMD simulations, C-OH2+ bond length observed to be smaller in water than in THF for the reason of higher stabilization of the protonated hydroxyl group in water due to its high polarity. The effect of two solvents on the reaction energetics is evaluated using CPMD-metadynamics reactions. Mechanism for the dehydration of HML-H+ is similar in both the solvents, that is, water removal, by the C-O bond cleavage, followed by the proton abstraction from β-carbon and formation of carbon-carbon double bond (Fig.1(B)). While in water, the average activation free energy barrier of TS is found to be around 133±8 kJ/mol, in THF, it is found to be ~107±9 KJ/mol (Fig.1(B)). The reduction of free energy barrier in THF depicts a relatively faster reaction in THF, and is found to be consistent with the experiments.
For the reaction in mixed solvent environment, we have first tried to understand the local solvation environment of the reactant using classical MD simulations for four different compositions of THF with mass fraction of 0.20, 0.40, 0.60 and 0.80 in water. Based on radial distribution functions for the oxygen atom of water in the vicinity of HML (center of mass), the water exhibits a higher tendency to solvate the hydroxyl group (Fig.1(C)). By motivating from this, we study the solvent effects on the acid catalyzed dehydration reaction on a broader level, protonated mevalonolactone (MVL) equilibrated in different solvents, water (polar protic), THF (polar aprotic) and cyclohexane (apolar) and in the mixture of these solvents, preliminary results showed the increase in water rich local domain near the reactant on increasing the organic phase.
Mechanistic insights are provided for the acid catalyzed dehydration of lactones in solvents of different nature and polarity, and their organization around the reactant can come up with an intimation to design the complex reaction environment for the conversion of biomass derived molecules.