2022 Annual Meeting
(42b) High-Silica Pd/H-LTA Catalysts for Low Temperature CH4 Oxidation
Authors
Tala Mon - Presenter, University at Buffalo
Jingzhi Liu, Syracuse University
Viktor Cybulskis, Syracuse University
Eleni Kyriakidou, SUNY at Buffalo
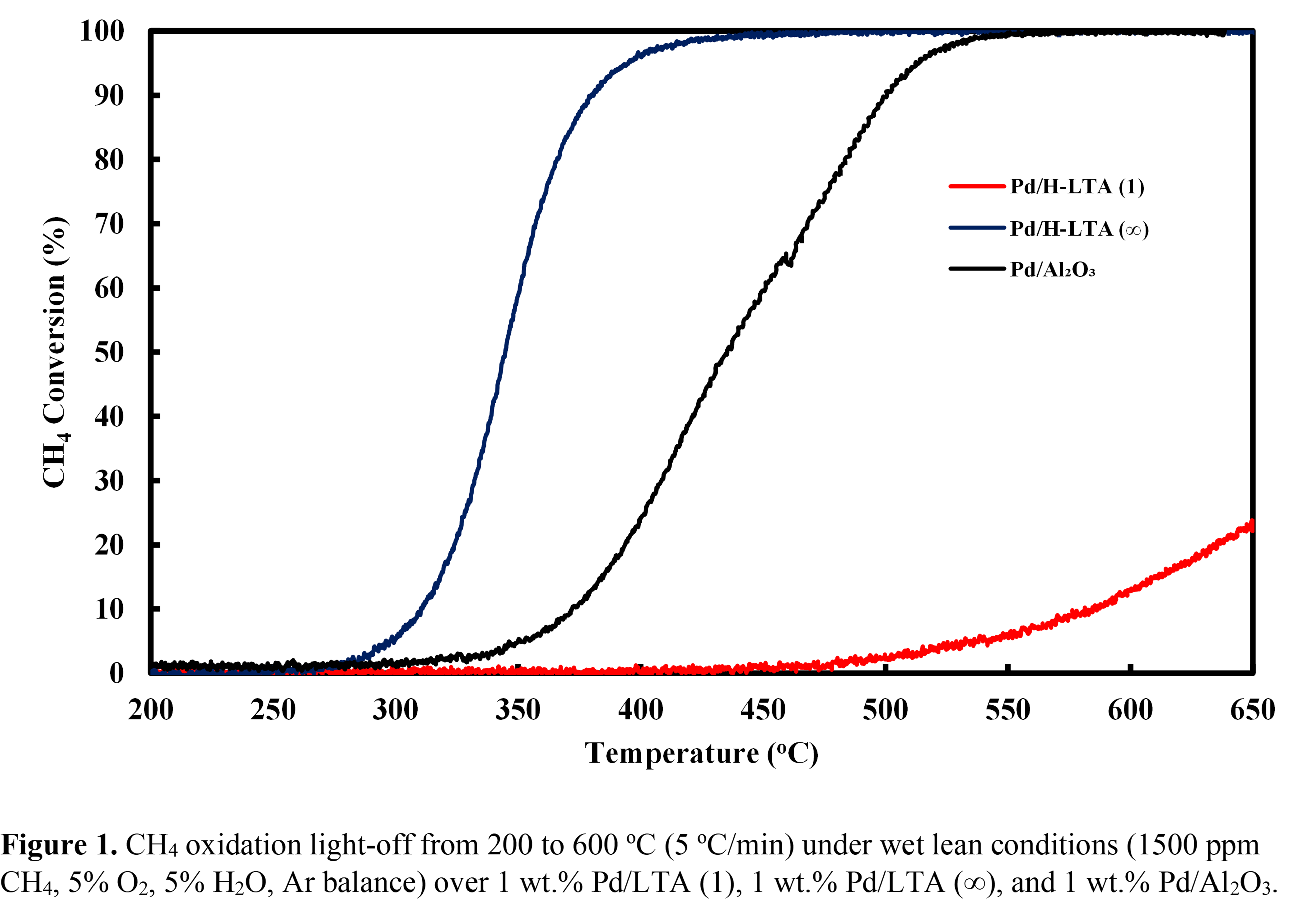
Natural gas has garnered attention as a cleaner alternative fuel for vehicles compared to gasoline or diesel. The main component of natural gas is methane (CH4) and is 25 times more potent than CO2 in impact to global warming over a 100-year period. The conventional solution for CH4 remediation is its catalytic oxidation using Pd/Al2O3 catalysts. However, Pd/Al2O3 catalysts suffer from low conversions and deactivation through Pd sintering in typical exhausts of natural gas vehicles containing high amounts of water vapor (5 â 10%) at temperatures < 400 °C. A promising support alternative is small-pore zeolites that are known for their hydrothermal stability and tunable hydrophobicity by varying the Si content to prevent water inhibition. Herein, small-pore H-LTA zeolites were synthesized with Si/Al molar ratios of 1 and â, loaded with 1 wt.% Pd, and evaluated for low temperature CH4 oxidation performance (1500 ppm CH4Â, 5% O2, 5% H2O, Ar balance). Pd/H-LTA (Si/Al = â) outperformed Pd/H-LTA (Si/Al = 1) and Pd/Al2O3 (Fig. 1). Pd/H-LTA (1) did not achieve temperatures required for 90% (T90) CH4 conversion up to 650 oC while Pd/H-LTA (â) converted CH4 at lower temperatures than Pd/Al2O3 with T90âs of 381 and 500 oC, respectively. Intermediate Si/Al molar ratios (15 and 34) of Pd/H-LTA are investigated to determine the full extent of the relationship between Si/Al and CH4 oxidation performance along with the apparent activation energies, CH4, O2, and H2O orders. Stability testing is performed at <10% CH4 conversion to determine the ability of the catalysts to maintain the CH4 oxidation performance and regain activity with the addition and removal of 5% H2O. Overall, the current trend suggests Pd/H-LTA (â) is optimum catalyst due to the hydrophobicity of the high-silica H-LTA support.