2022 Annual Meeting
(425b) Identifying Effects of Phosphorous in Transition Metal Phosphides for Selective Hydrogenolysis of Hindered C–O Bonds
Authors
David Hibbitts, University of Florida
Hansel Montalvo-Castro, University of Florida
Abdulrahman Almithn, University of Florida
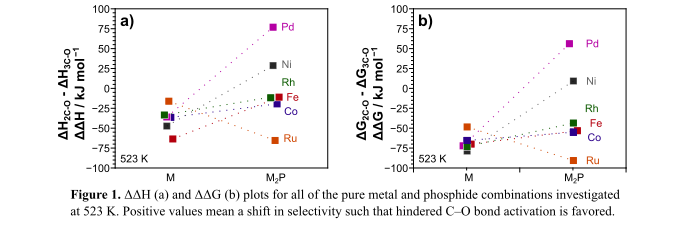
For biomass-derived molecules, 2-methyltetrahydrofuran (MTHF) is a model compound that can be used to contrast hindered and unhindered CâO bond activation. MTHF contains a furan ring whose O atom has neighboring secondary (2CâO) and tertiary (3CâO) carbons. Supported Ni catalysts activate the unhindered 2CâO bond at a rate 10Ã that for hindered 3CâO bonds in MTHF, as supported by DFT-derived ÎHact values on Ni(111) surfaces that are 47 kJ molâ1 lower for 2CâO bond activation. This selectivity shifts toward 3CâO activation as phosphorous is incorporated, with ÎHact for 3CâO bond activations that are 11 kJ molâ1 and 29 kJ molâ1 lower on Ni12P5 and Ni2P, respectively [1]. It remains unclear how P causes these shifts in selectivity, which could occur because of geometric (separation of Ni centers) or electronic effects (withdraw of eâ from Ni). Here, we use DFT calculations to study the effect of P incorporation into other transition metals using surfaces isostructural to Ni(111) and Ni2P(001) to decouple electronic from geometric effects. Our results show that incorporating P on a series of transition metals consistently shifts selectivities toward 3CâO activation, reflected by the difference in the 2CâO and 3CâO bond activation enthalpies (ÎÎH) and free energies (ÎÎG): Ni2P, Pd2P, Rh2P Fe2P and Co2P all have more positive values of ÎÎH and ÎÎG than their pure metal counterparts (Fig. 1). The most dramatic effects of P incorporation are observed in Ni2P and Pd2P, and only those two surfaces had lower 3CâO activation barriers than those for 2CâO. These data begin to provide insights into the geometric and electronic effects of P incorporation which appear to cooperatively increase the selectivity toward hindered 3CâO bonds.
References