2022 Annual Meeting
(41c) Reaction Driven Restructuring of PdCu Bimetallic Catalysts and Consequences for Selective Dehydrogenation and Oxidation Catalysis
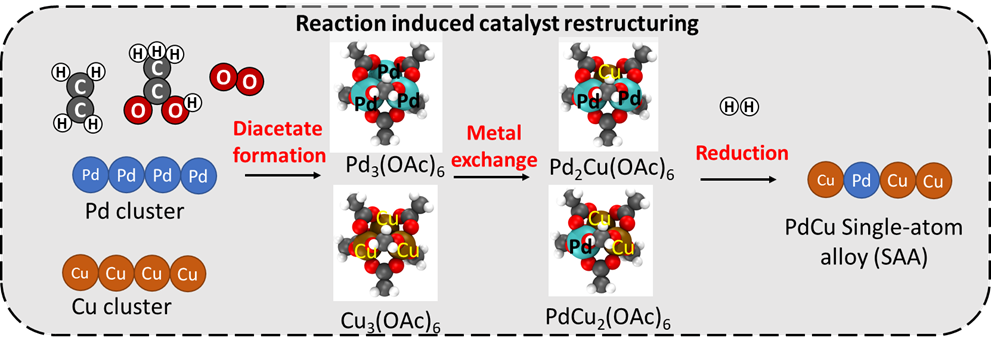
The SAA formation is probed by CO-IR, confirming the elimination of Pd-Pd bridge bonded CO, and XAFS, showing high Pd-Cu coordination and no Pd-Pd coordination. The restructuring mechanism for the SAA formation is probed using in-situ IR and comparison with DFT predicted spectra and stability of different diacetate species.
The catalytic consequences of the SAA formation are tested using ethanol dehydrogenation at 473 K (2kPa ethanol in He, 40cc/min). The catalyst shows moderate ethanol dehydrogenation rate (18 Pd-1ks-1) and selectivity (70%) before the vinyl acetate reaction induced restructuring. However, after the restructuring, the same sample shows much higher rate (40 Pd-1ks-1) and selectivity (> 99.8% at 75% ethanol conversion), which is consistent with the suppression of C-C cracking by the elimination of Pd-Pd pairs. The rate and selectivity for these SAAs is higher than that for co-impregnated PdCu bimetallic catalysts with the same loading (32 Pd-1ks-1 and 98%). These results suggest that the reaction induced restructuring shown here is an efficient method for SAA formation without involving more complex colloidal and galvanic replacement methods. The physical-mixtures catalyst also exhibits much higher VA synthesis rates and selectivity than Pd at low oxygen pressures. These results reveal a facile method and mechanism for SAA formation and the resulting improved catalytic performance, which may have implications for many systems beyond the specific example used here.