2022 Annual Meeting
(374e) Optimal MEA/Dipa/Water Blending Ratio for Minimizing Regeneration Energy in Absorption Based Carbon Capture Process: Experimental Solubility and Thermodynamic Modeling
Authors
Kyung-Min Kim, Gangneung-Wonju National University
Jong-Ho Moon, Chungbuk national university
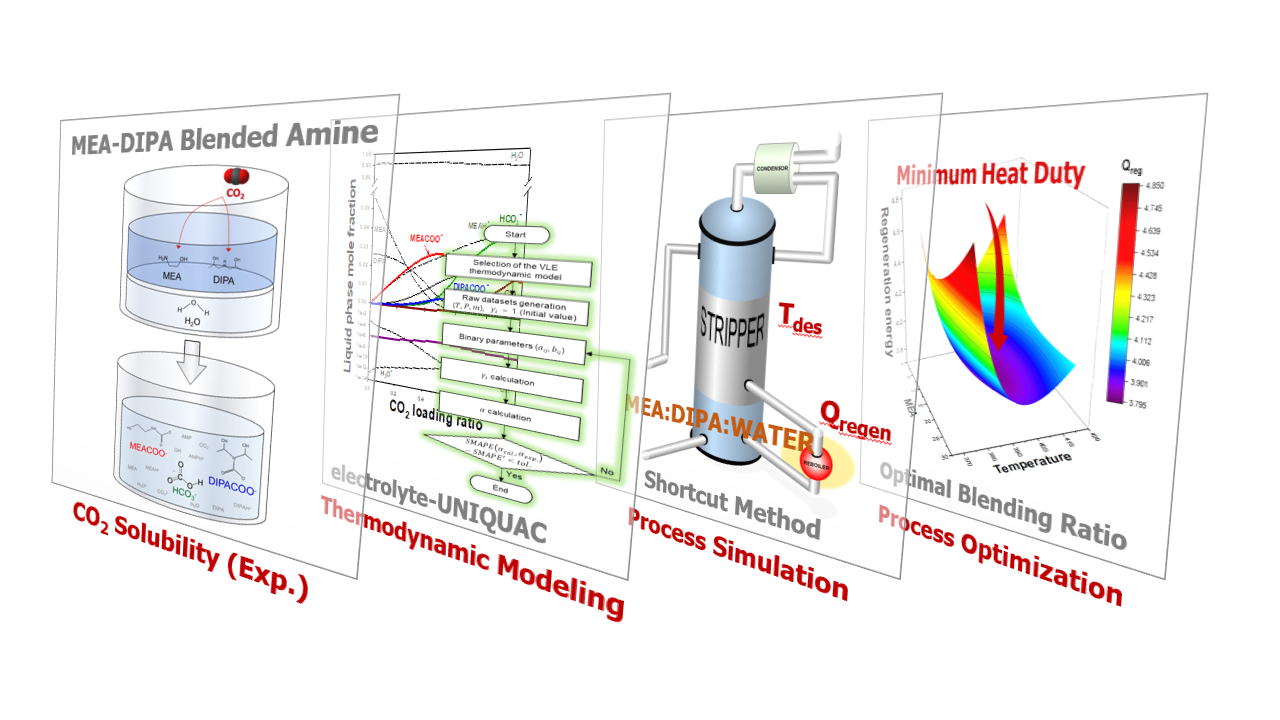
A new CO2 absorption solution was developed by blending monoethanolamine (MEA) and diisopropanolamine (DIPA) in H2O. The solubility of CO2 in MEA/DIPA aqueous solutions with various blending ratios was measured at a temperature range of 323.15â383.15 K under CO2 partial pressures of up to 400 kPa. The successive substitution method was introduced to calculate the molar fractions of all 12 species, and the electrolyteâUNIQUAC model was applied to consider non-idealities. Then, the optimal blending ratio was obtained for four different targets: 1) max Îð¼ð¶ð2 (total amine-based CO2 cyclic capacity), 2) max Îð½ð¶ð2 (total solution-based CO2 cyclic capacity), 3) min âÎhabs (heat of CO2 absorption under stripping condition), and 4) min Qregen (regeneration energy for the carbon capture process). Here, Îð¼ð¶ð2, Îð½ð¶ð2, and âÎhabs are the properties of solution, while Qregen is a performance parameter of the process. The Qregen, the sum of sensible heat, latent heat, and heat of reaction, was calculated according to the amine blending ratio using the âshortcut methodâ [1]. In the process, besides the cyclic capacity and heat of absorption, the effect of sensible heat and latent heat must be considered simultaneously. Therefore, the most reliable method to determine the amine blending ratio is Qregen minimization. The obtained minimum Qregen of the process was 3.44 GJ/t CO2 using MEA/DIPA/H2O solutions of 4.01/25.99/70 (w/w/w), which was 8.7% and 2.6% lower than those of the single MEA and DIPA aqueous solutions due to the synergistic effect of MEA and DIPA.