2022 Annual Meeting
(360bg) Investigating Stable and Active Catalysts for Hydrogen Generation Via Methane Pyrolysis in Molten Media, Using Ab Initio Molecular Dynamics
Authors
Ojus Mohan - Presenter, Nanyang Technological University
Samir H. Mushrif, University of Alberta
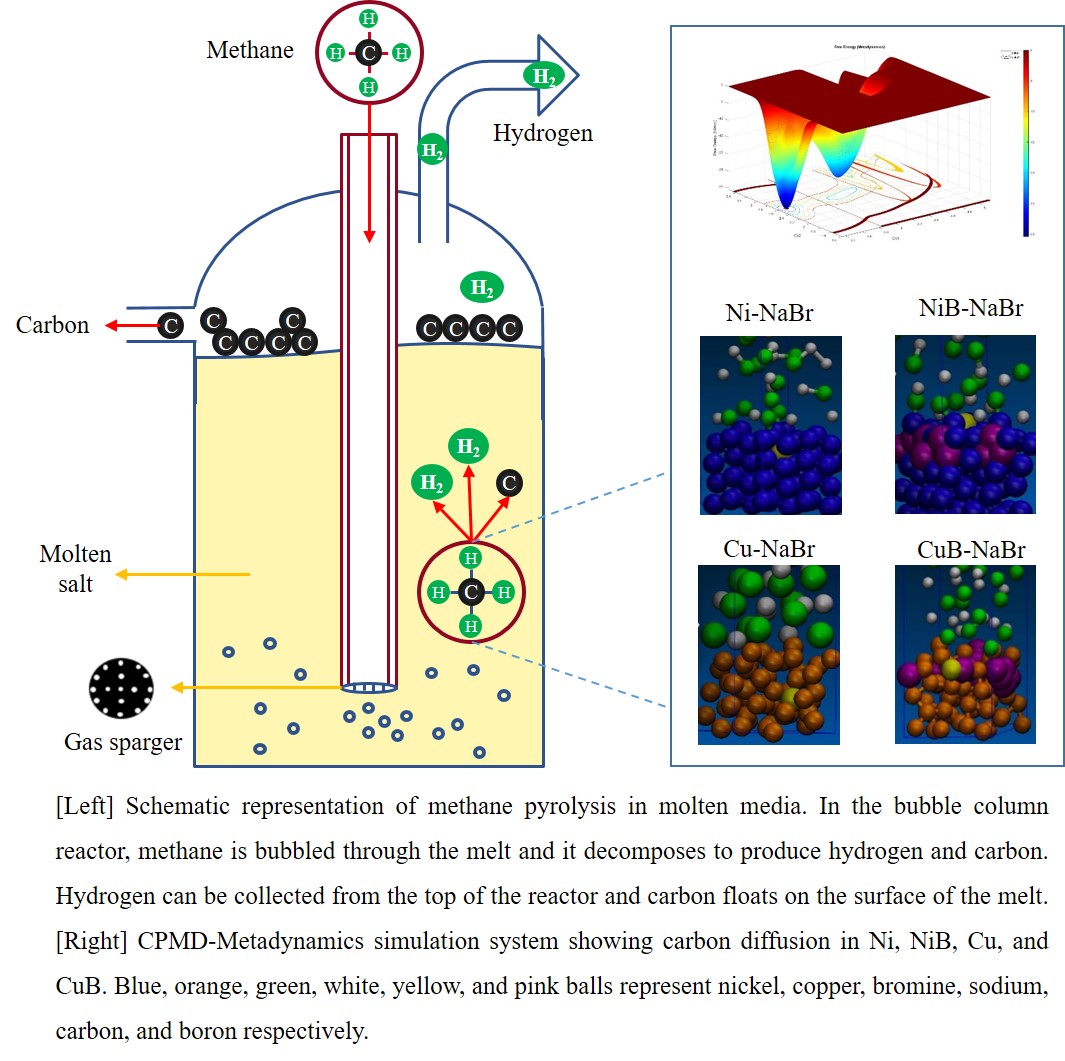
Hydrogen has the potential to replace fossil fuels as a sustainable energy carrier in a variety of applications. Methane pyrolysis (CH4(g)âC(s)+2H2(g)), enables the possibility to produce CO2-free hydrogen from natural gas. Since methane is a highly stable molecule with strong C-H bonds, a catalyst is required to overcome kinetic limitations and achieve industrially viable reaction rates. Previous attempts to employ solid metal catalysts failed due to the catalytic deactivation caused by carbon formation on the surface and/or its diffusion into the bulk of the catalyst. Remarkably, the deactivation of the catalyst can be avoided by performing the reaction in molten salt since the carbon floats on the molten salt surface. Alternately, if C-C coupling can be facilitated, we may fully eliminate carbon formation and obtain more valuable C2 products, along with hydrogen. However, most salts are catalytically less active compared to metals, requiring extremely high temperatures to decompose methane molecule and they cannot facilitate C-C coupling either. In this work, the objective is to enhance the catalytic performance of molten media without compromising its stability, and to facilitate C-C coupling by dispersing metal catalysts (Ni,Cu) into salt. We employed ab-initio molecular dynamics simulations combined with the accelerating approach metadynamics, which enables simulating reaction dynamics at a finite temperature and computing free energy barriers along the minimum energy pathway. We discovered that i)carbon diffused into Ni and Cu, leading to catalyst deactivation and that ii)boron doping altered the microstructure of Ni and Cu, preventing carbon diffusion into the catalyst. We further investigated C-C coupling reactions in pure and boron-doped metal-salt systems and compared the energetics for sequential methane dehydrogenation and C-C coupling. The mechanistic insights revealed in this study will aid in the development of active and stable molten media based systems for methane pyrolysis, paving the way for its commercialization.