2022 Annual Meeting
(30g) Water-Soluble Spiropyran Copolymers for Reversible Light-Induced Transition Metal Complexation and Removal
Authors
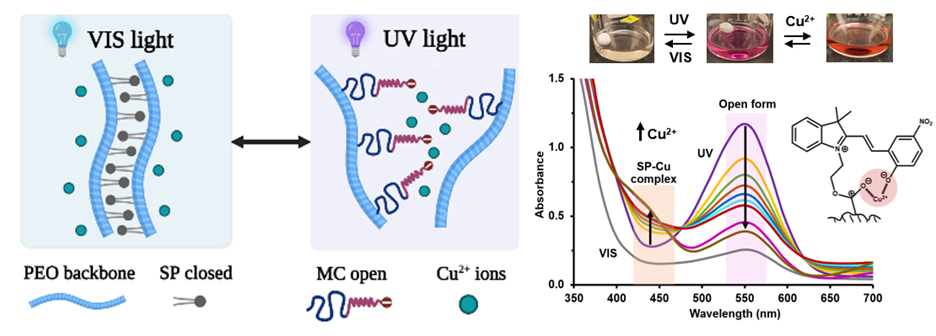
Previous research on spiropyran has demonstrated its complexation behavior in organic solvents. To enable its applicability in water treatment, spiropyran (SP) acrylate and methoxy-spiropyran (SP-OCH3) acrylate monomers were copolymerized with polyethylene glycol (PEO) methyl ether acrylate to impart solubility and selective metal-ion complexation in aqueous solutions. The structure and loading percentage of spiropyran moiety in the copolymer were confirmed using 1H NMR. UV-VIS spectrometric studies and ICP-MS were utilized to analyze and quantify the reversibility and coordination behavior of the polymer with copper (II) ions.
The zwitterion formation and copper complexation under irradiation of UV light exhibit fast kinetics following a pseudo-first order behavior. A featured band at 550 nm corresponding to the open zwitterionic (merocyanine MC) form undergoes a reduction in absorbance with an increase in the concentration of copper ions. This is followed by the formation of a new band at 430 nm which represents the SP-Cu complex. Upon irradiation with visible light, these absorbance bands disappear indicating the breakage of the complex as the SP moiety undergoes a ring closure. The reappearance of the absorption bands over multiple cycles of light irradiation confirmed the reversible ring opening and closing of the SP. Furthermore, ICP-MS measurements were used to quantify the copper removal from water. A solution of 10 mg/mL PEO-co-SP (3 mol% SP) polymer in water at a pH of 6.0 enabled the removal of 70% of the copper added. Further experiments will be carried out to study the effects of the loading percentage of SP moiety in the copolymer as well as an additional methoxy binding site for the improved selectivity and reversibility of SP-Cu coordination.