2022 Annual Meeting
(2ej) Understanding the Effect of Nanoconfinement on Carbon Dioxide Reaction with Water Using Reactive Molecular Dynamics Simulations
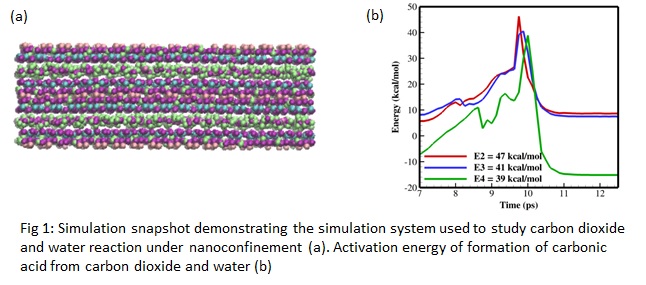
Carbon dioxide (CO2) is the major constituent of the greenhouse gases and the main contributor to global warming across the world. It is produced from various sources, including biomass energy installations and fossil fuel combustions. The concentration of CO2 in the atmosphere has increased from 270 ppm in pre-industrialization to nearly 400 ppm at present. We can mitigate the climate change by removing CO2 from atmosphere, and then store, sequester, or re-use it. These processes might involve the interaction, including the chemical reaction, of water with CO2 in nanoporous materials. Here, we apply molecular dynamics simulations using ReaxFF force field [1] to understand the reaction dynamics of CO2 and water in bulk and under clay nanoconfinement (e.g., Fig. 1a). Clay minerals [2] such as montmorillonite are effective minerals for sorption due to their superior thermal, mechanical, and chemical properties. We study the effect of confinement on the conversion of carbon dioxide to bicarbonate and carbonate ions. We perform free energy calculations using metadynamics [3] to understand the conversion mechanism of CO2 to weak carbonic acid and thereby soluble bicarbonates and carbonates. Using bond boost ReaxFF [4], we also calculate the activation energy (e.g, Fig. 1b) of each reaction steps to understand the likelihood of formation of carbonic acid in different environment. Our study provides a mechanistic understanding of the CO2 conversion under nanoconfinement.
Research Interests
Molecular dynamics simulations, Density Functional Theory, Free Energy Calculations, Interatomic Potential Development, Machine Learning, Deep Learning, Continuum and mesoscale modelling
Teaching Interests
Chemical Engineering Thermodynamics, Atomistic Scale Modeling and Simulations, Transport Phenomena, Chemical Reaction Engineering, Statistical Thermodynamics