2022 Annual Meeting
(272b) Digital Twin Development for Continuous Drug Manufacturing
Authors
Busuyi Adebayo - Presenter, Missouri University of Science & Technology
Syed Ahmed, Biogen
Rob Guenard, Biogen
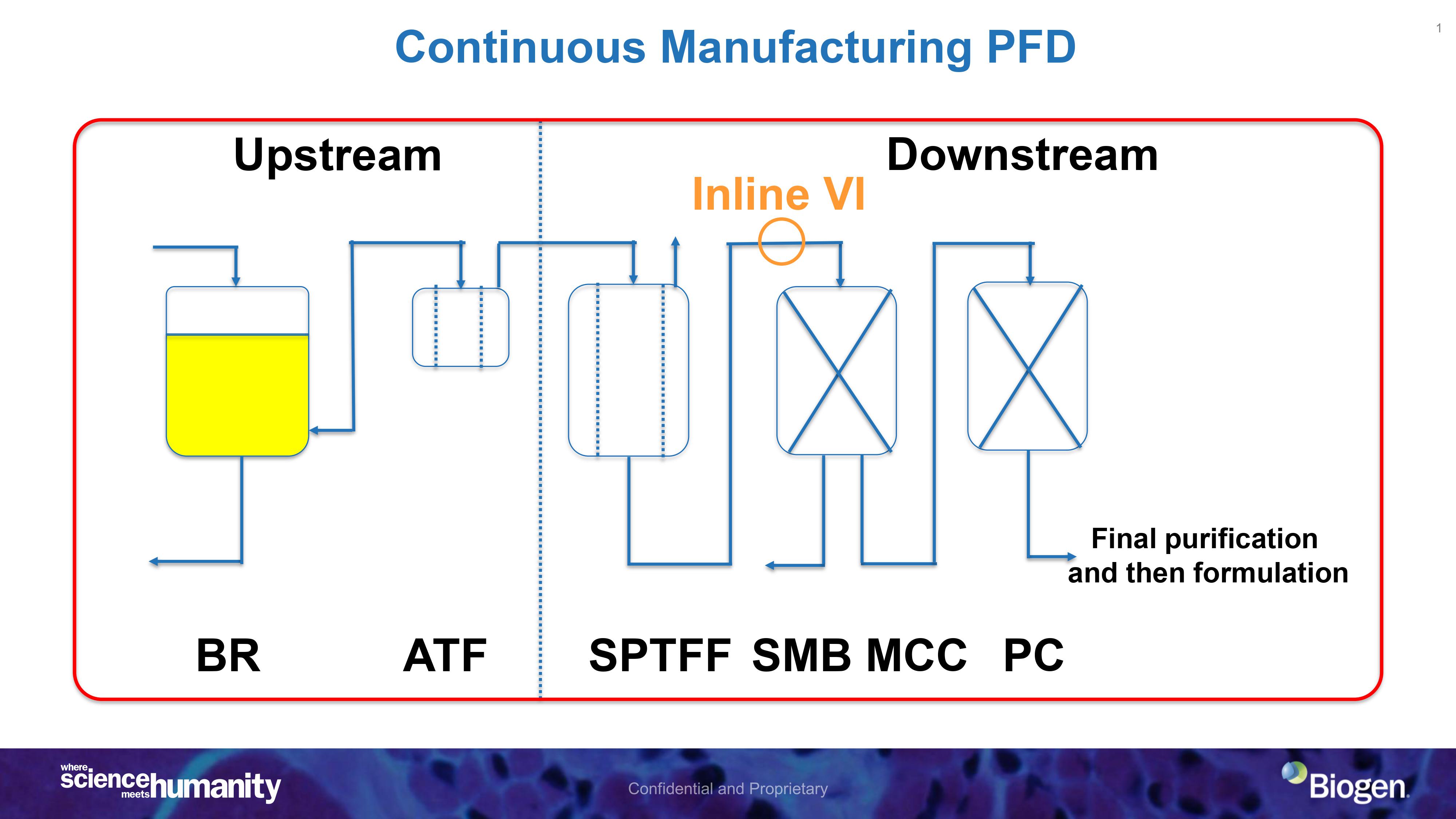
Rising above numerous setbacks and inherent challenges, biopharmaceutical industry has started to consider the integration of upstream and downstream operations for continuous drug manufacturing to shore up drug supply chain, reduce (cross)contamination, and time to market to mention but a few. For successsful development of this in intricate processes of drug manufacturing, mechanistic modeling and simulation has been recommended by subject matter experts, policy makers and regulation agencies such as FDA and EMA.
Herein, we focus on:
1. Mechanistic modeling and simulation of the unit operations in a typical continuous drug manufactuirng train