2022 Annual Meeting
(253i) Antibody-Lectin Bispecifics for Glyco-Immune Checkpoint Blockade
Authors
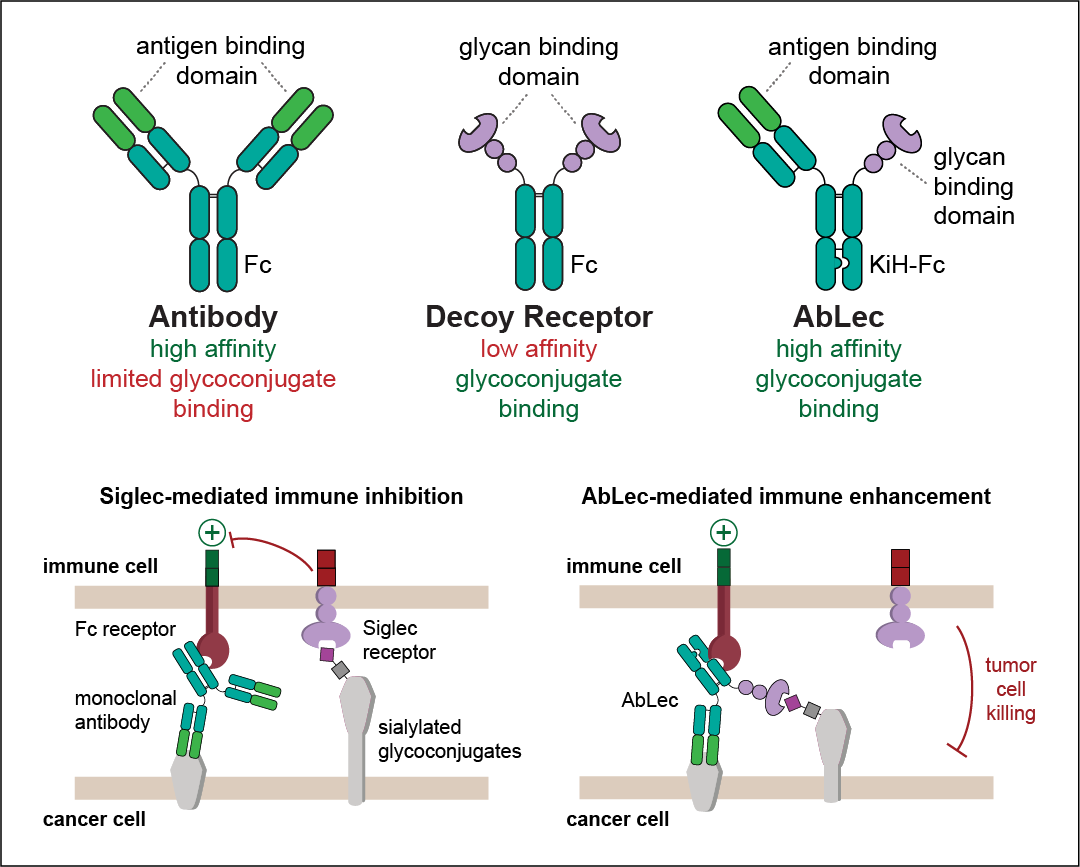
To address this need, we have developed a new class of antibody-lectin bispecifics (AbLecs) targeting tumor-associated glycans for checkpoint blockade. In this approach, glycan-binding domains from immune receptors (e.g., Siglec receptors) are coupled to high-affinity binding domains from FDA-approved antibodies targeting common tumor-associated antigens (e.g., trastuzumab) via knobs-into-holes heterodimerization technology. Western blot and mass spectrometry results indicated correct heterodimeric assembly of eight AbLec candidates, demonstrating the feasibility of the AbLec approach. We found that AbLecs bind cancer cell lines at nanomolar concentrations and can block cognate Siglec receptor binding via flow cytometry. Finally, we showed that AbLecs enhance killing of diverse human tumor cell lines by primary immune cells in vitro compared to their FDA-approved parent antibodies. Enhancement of in vitro tumor cell killing with AbLecs was dependent on the targeted Siglec and was more potent than the combination of the parent monoclonal antibody and a Siglec-blocking antibody. These studies provide proof-of-principle for AbLecs as a first-in-class, modular platform technology enabling blockade of glyco-immune checkpoints for cancer immunotherapy.