Selective hydrogenolysis of organic molecules with heteroatoms plays a large role in hydrogen-treating molecules from biomass (O, N removal), petroleum (S removal), wastewater (Cl removal), and pharmaceuticals (O, N, Cl removal). Kinetic and density functional theory (DFT) studies have shown that hydrogenolysis of saturated CâC bonds occurs
via unsaturated CH*âCH* intermediates on various Pt-group metals.
1 Kinetic and DFT studies of 1-propanol and 1-butanol on Ir, Pt, Ru, and Cu directly contrasted CâO hydrogenolysis, CâC hydrogenolysis, and decarbonylation.
2 Decarbonylation was favored on all Pt-group metals, followed by CâO hydrogenolysis with CâC hydrogenolysis occurring at the lowest rate, except on Cu, where CâO hydrogenolysis was favored over competing CâC activation reactions.
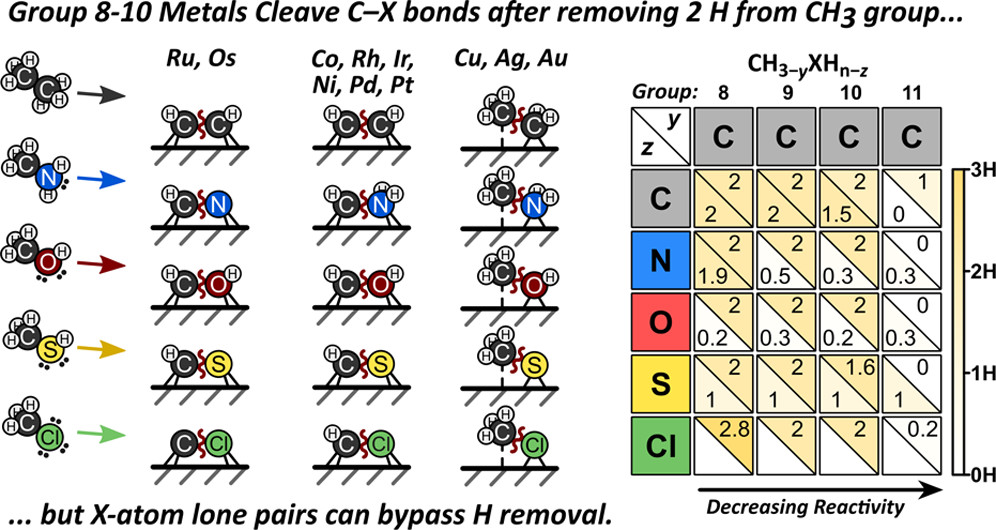
2 Our recent study of saturated CH
3XH
n molecules (X = C, N, O, S, Cl) on group 8â11 transition metals have shown that when cleaving, the heteroatom and metal identities affect the predicted level of unsaturation of the transition state.
3 Free energy barriers indicate that CâX activations on group 8â10 transition metals typically occur after removing two H atoms regardless of heteroatom identity, whereas hydrogenolysis on group 11 transition metals occurs
via more saturated intermediates.
3 Metal identity determines dehydrogenation of âNH
2 prior to CâN activation. Dehydrogenation of âOH was rare while dehydrogenation of âSH was universal.
3 We have since extended prior study to assess the mechanisms that activate PhâX in substituted aromatic species. In addition to varying heteroatom identity, we vary hydrogenation of the ring moiety by systematically adding and subtracting H atoms. In general, understanding the mechanisms of heteroatom removal from simple organic molecules furthers fundamental insight into hydrogenolysis of more complex organic molecules.
References:
- J Catal., 2014, 311, 350â356
- Am. Chem. Soc., 2015, 137, 11984â11995
- ACS Catal., 2020, 10, 5086â5100