2022 Annual Meeting
(177f) Synthesis of Well-Defined IrO2/TiO2 Catalysts for Low-Temperature Activation of Methane
Authors
Helena Hagelin Weaver - Presenter, University of Florida
Bochuan Song, University of Florida
Li-Yin Hsiao, University of Florida
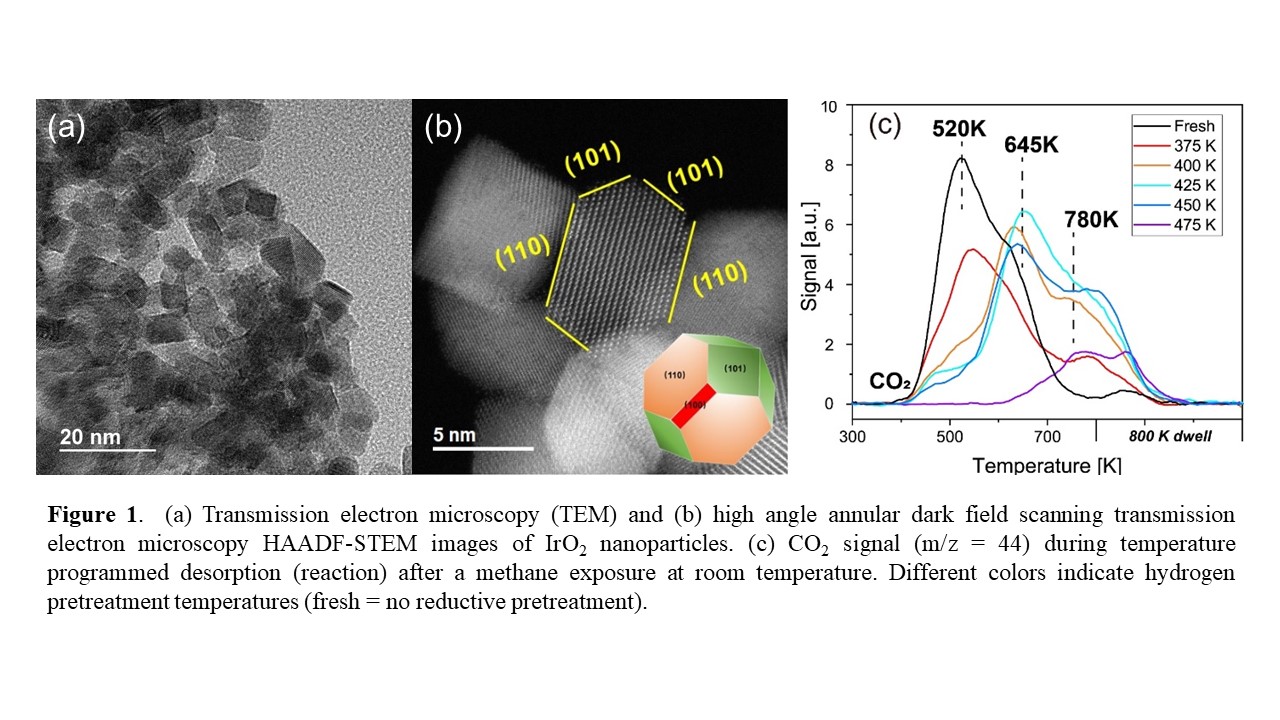
Previous work has shown that IrO2(110) surfaces are very active in the low-temperature activation of methane. Inspired by this work, we synthesized IrO2 nanoparticles and IrO2 supported on rutile TiO2 nanorods and investigated their activity towards methane activation at room temperature. The synthesis conditions yielded IrO2 nanoparticles approximately 8 nm in diameter and they were shown to expose a significant fraction of the desired (110) surface facets (Figure 1a and b). After an oxygen treatment to convert any Ir2O3 to IrO2, and then outgassing to remove excess oxygen, the IrO2 nanoparticles were exposed to methane at room temperature. After removing gas phase methane, the IrO2 nanoparticles were heated in inert to 800 K while monitoring the products released. These experiments revealed that the IrO2 nanoparticles do indeed activate methane at room temperature, as carbon dioxide desorbed around 520 K. The effect of a mild reduction (between 375 to 475 K) was also evaluated and revealed a slight increase in the overall amount of methane activated up to a reduction treatment of 425 K, as oxygen removal exposed additional sites. However, the CO2 desorption peak also shifted to higher temperatures, revealing that the remaining oxygen was more difficult to remove.
To decrease the IrO2 content, TiO2-supported IrO2 catalysts with different IrO2 loading were also synthesized using an urea precipitation method. A synthesis method for rutile TiO2 nanorods were selected to maximize the (110) surface facets exposed. Even at a 10% iridium loading (by weight) on the TiO2 support, the signals obtained from the IrO2/TiO2 catalysts are significantly lower compared with the pure IrO2 but indicate that the supported catalyst can also activate methane at room temperature.