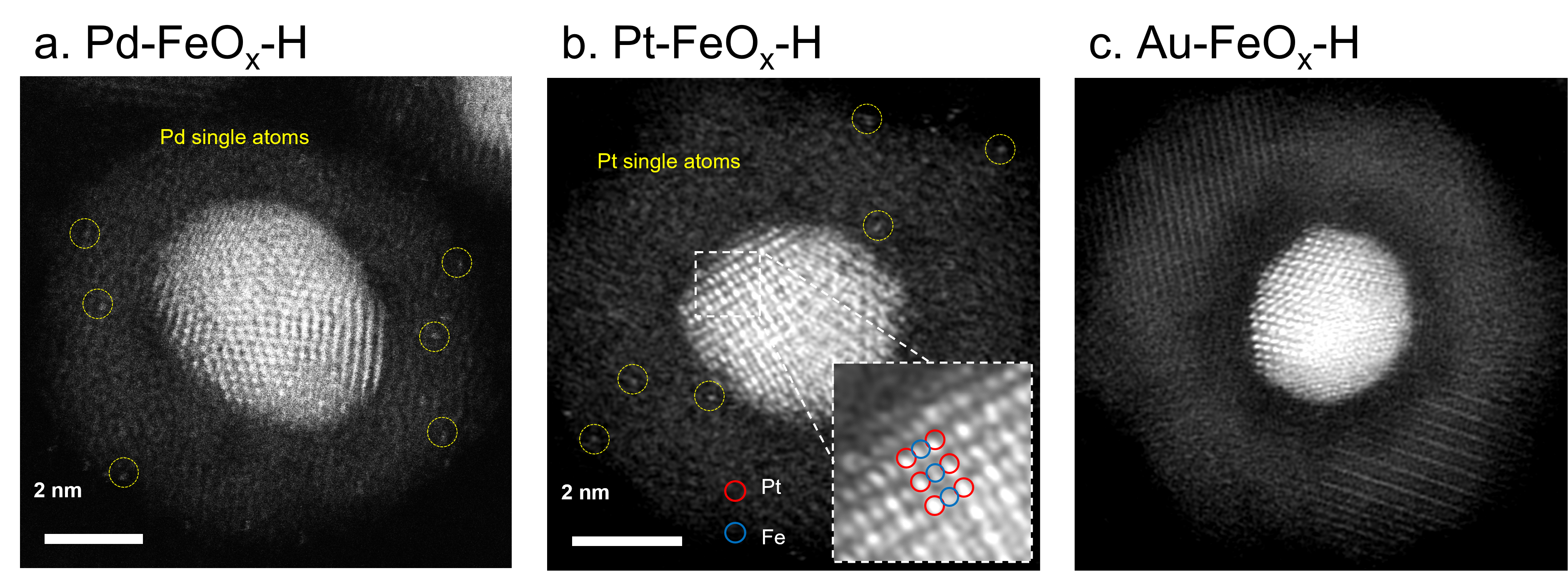
Metal-metal oxide interactions play an important role in heterogeneous catalysis, and therefore, have spurred tremendous interest in the in-depth understanding and investigation of the strength, stability, and dynamic evolution of such interactions. Herein, we synthesize a series of core-shell metal-iron oxide nanocrystals (M-FeO
x, M = Pd, Pt, Au) and employ both in situ and ex situ electron microscopy and X-ray absorption spectroscopy (XAS) to study the atomic and nanoscopic dynamic M-FeO
x interactions under reductive atmosphere (H
2) at elevated temperatures. The H
2-treated M-FeO
x core-shell structures all evolved into yolk-shell structures (denoted as M-FeO
x-H). However, dissimilar metal-metal oxide interactions have been observed among the three M-FeO
x-H systems. We show that Pd core interacts strongly with the FeO
x shell after the H
2 treatment, featured by the formation of Pd single atoms on the FeO
x shell and increased Pd-Fe bonding. Meanwhile the Pt core transforms into an ordered PtFe intermetallic structure and Pt single atoms are anchored on the FeO
x shell immediately upon the coating of FeO
x. The PtFe intermetallic core is thermodynamically stable without bonding change during the post-synthesis H
2 treatment. In contrast, Au-FeO
x-H forms negligible Au-Fe bonding nor Au single atoms. Finally, we conduct comprehensive catalytic tests of M-FeO
x-H systems on acetylene semihydrogenation reaction. Among the three systems, Pd-FeO
x-H exhibits best catalytic performance, achieving 100% acetylene conversion and 86.5% ethylene selectivity at 60 °C. Our work not only depicts the dynamic evolution of the metal-metal oxide interactions of M-FeO
x systems but also offers a unique method to synthesize yolk-shell nanocomposites consisting single atoms and intermetallic alloys. The resulting M-FeO
x-H yolk-shell nanocrystals can be employed as excellent catalyst for reactions including but not limited to alkyne hydrogenation.