2022 Annual Meeting
(176f) Effect of Water Concentration on Rates and Selectivities of Alkene Epoxidations in Ti-BEA Zeolites
Authors
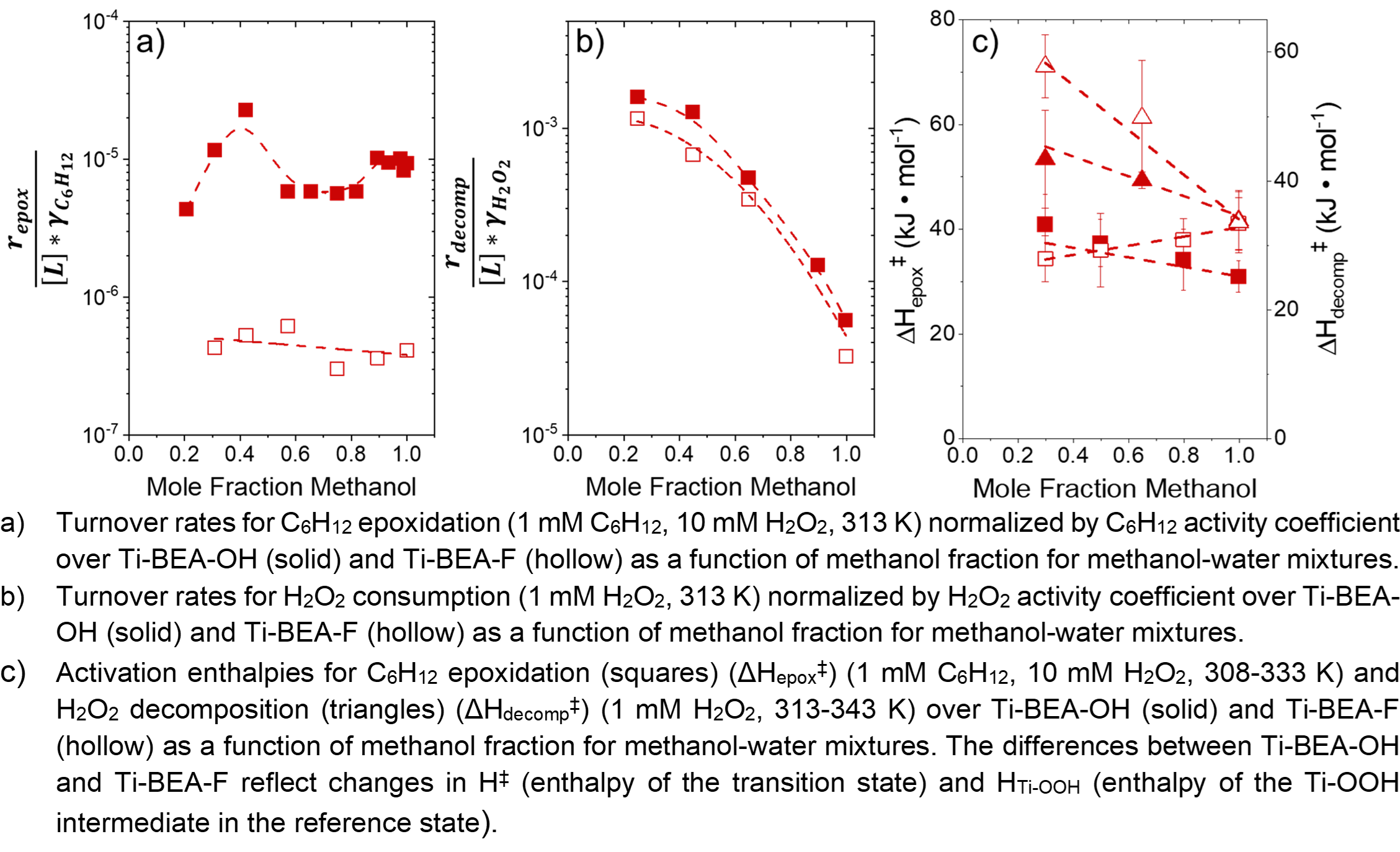
As the mole fraction of H2O increases from 0.002 to 0.8 in acetonitrile, gamma-butyrolactone (GBL), and methanol co-solvents, turnover rates for 1-hexene (C6H12)epoxidation increase while H2O2 decomposition rates decrease. The inverse trends lead to significantly higher epoxidation selectivities at higher H2O fractions. Rates were normalized by reactant activities to show how changes in the stability of the Ti-OOH MARI and transition state contribute to rate differences. As H2O fraction increases, activation enthalpies for epoxidation (ÎHepoxâ¡) change monotonically over Ti-BEA-F but non-monotonically over Ti-BEA-OH for each co-solvent. ÎHepoxâ¡ differences result from changes in the stability of reactive species within the pores of Ti-BEA-OH and Ti-BEA-F. Corresponding measurements of 1,2-epoxyhexane (C6H12O) adsorption enthalpies with isothermal titration calorimetry correlate to ÎHepoxâ¡ measurements, providing further evidence that [H2O] affects epoxidation transition state stability. Activation enthalpies for H2O2 decomposition (ÎHdecompâ¡) show that adding H2O leads to enthalpic stabilization of the transition state, but the corresponding stabilization of liquid-phase H2O2 suppresses decomposition. H2O molecules stabilize the hydrophilic transition state for H2O2 decomposition through hydrogen bonding. In contrast, H2O likely entropically stabilizes the hydrophobic epoxidation transition state, increasing ÎSepoxâ¡ through the disruption of hydrogen bonds between H2O. These results show that upon adding more H2O, enthalpic effects suppress H2O2 decomposition while entropic effects facilitate epoxidation. Collectively, these findings show that adding H2O to organic solvents provides opportunities to increase turnover rates and selectivities for desired reaction pathways.