2022 Annual Meeting
(124d) Substituted Zeolites As Promising O2 Sorption Pump Materials: A Density Functional Theory Study
Authors
Steven Wilson - Presenter, Arizona State University
Ellen B. Stechel, Sandia National Laboratories
Ivan Ermanoski, Arizona State University
Christopher L. Muhich, University of Colorado at Boulder
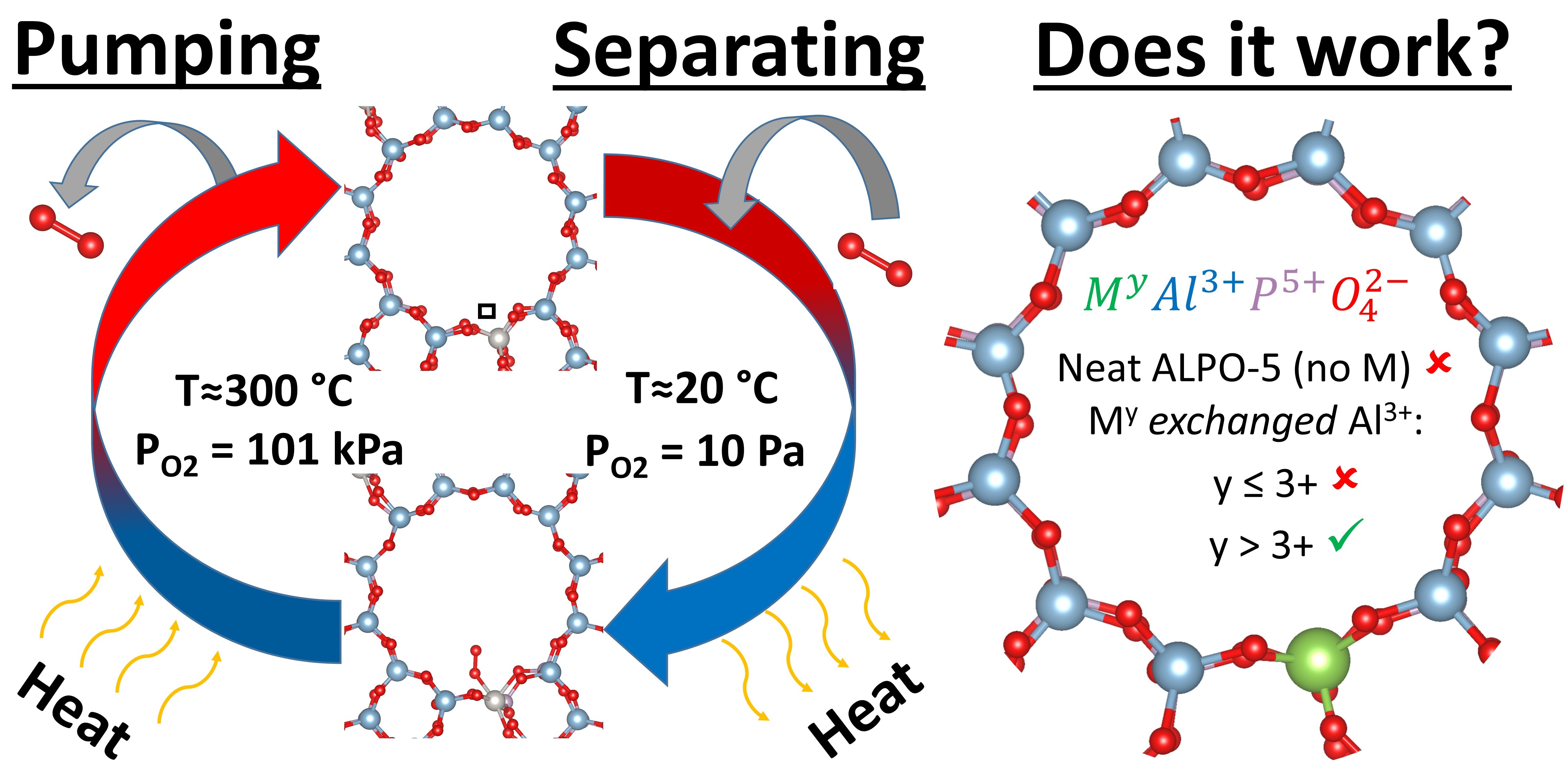
Many chemical processes depend on having an environment that is low in oxygen partial pressure (PO2 < 100 Pa); sorption pumps are a promising route to establishing that environment by either oxygen pumping or oxygen separation from an inert gas. Near ambient sorption-based processes rely on either pressure or thermal swings, requiring no moving parts, no electricity, and neither very high nor very low temperatures. In this work, we use ab initio calculations to explore zeolites as a class of materials for sorption-based oxygen pumping/separation. Our calculations indicate that while the neat AlPO4 composition of ALPO-5 zeolite (IZA-code AFI) does not adsorb O2, O2 does adsorb on zeolites selectively substituted with transition metals and metalloids and hence, can enable separation and pumping. ALPO-5 substituted with Si, Ge, Sn, Pd, Pt, Ti, V, Cr, Mn, Zr, Mo, Hf, W, Ce, and Pr provides adsorption energies ranging from -0.19 to -3.92 eV, (-) indicates exothermic process. Additionally, we provide a comprehensive understanding of what controls the adsorption energy: 1) the substitutions must be able to adopt an oxidation state that is more positive than the cation it replaces, and 2) the size of the pore into which the O2 adsorbs to the wall. We compare these trends found in substituted ALPO-5 to three other AlPO4 compositional zeolites VPI-5, SSZ-51, and ALPO-52, IZA-code VFI, SFO, and AFT respectively. We find that the factors in controlling binding energy are confirmed in these three zeolites as VPI-5, which contains on pore ~3 Å larger in diameter, substituted with similar elements results in higher binding energies than ALPO-5. Furthermore, ALPO-52 having smaller pores than ALPO-5 is less favorable to O2 binding. Lastly SSZ-51s structure is similar to ALPO-5s, with only a 0.5Å dimeter difference in the largest pore, and results in almost identical O2 binding behavior. Additionally, we conduct a thermodynamic analysis of a thermal swing cycle to approximate the optimal O2 binding energy for low energy O2 pumping/separation. We find that the minimum energy cost likely occurs when the adsorption energy is in the range of 0.75 â 1.00 eV (72 â 97 kJ·mol-1), which corresponds to Ge, V, Pt, or Ce substituted ALPO-5. While also investigating the thermodynamic selectivity towards O2 adsorption as compared to other air molecules like N2, CO, CO2 etc.