2020 Virtual AIChE Annual Meeting
(712e) Critical Role of Dispersive Transport during Absorptive Hydrogen Removal for Enhanced Aromatics Yield in Methane Dehydroaromatization on Mo/H-ZSM-5 Catalysts
Authors
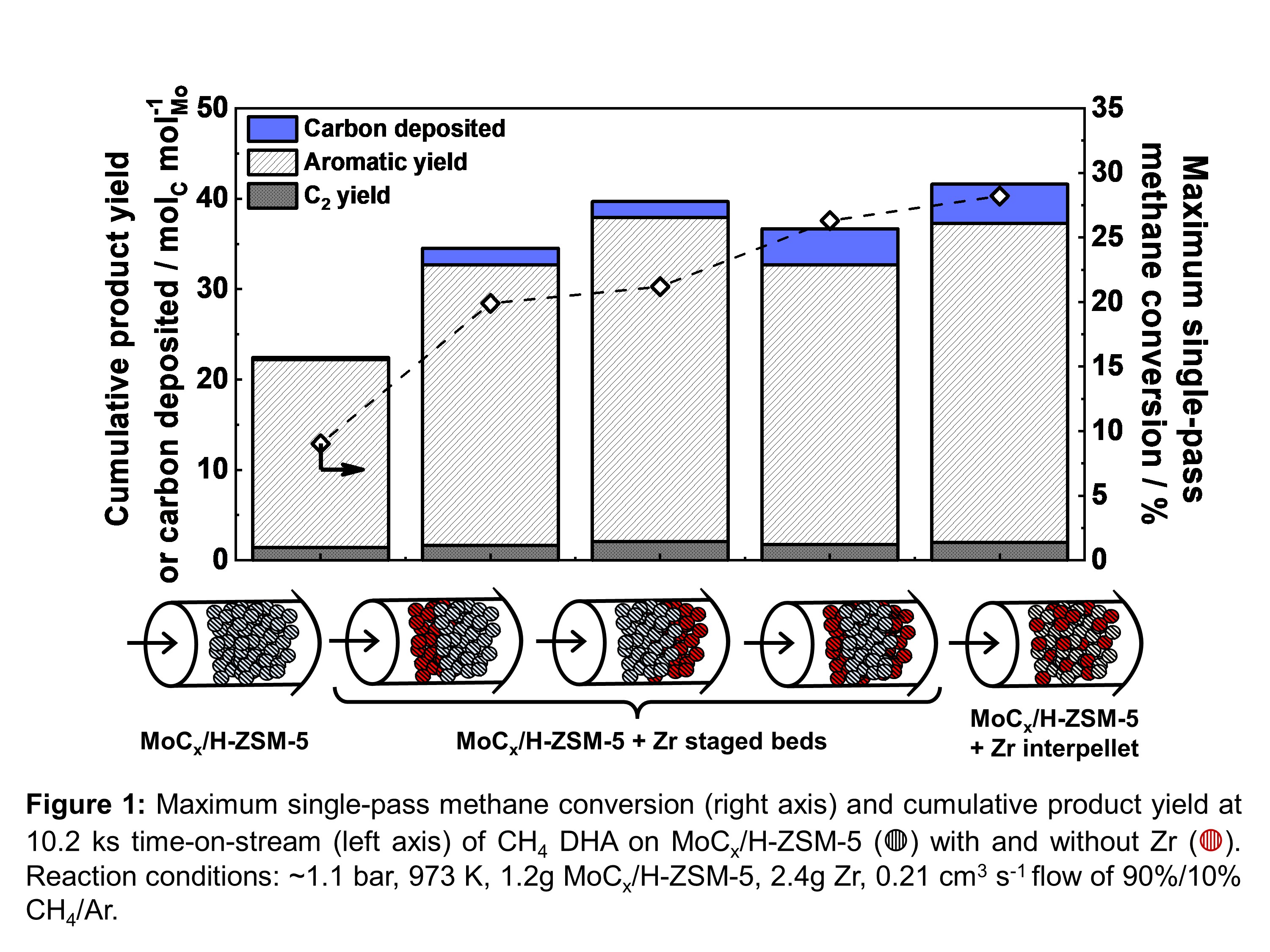
The efficacy of staged MoCx/H-ZSM-5 and Zr beds to circumvent DHA thermodynamic limits is attributed to the dispersive conveyance of gas-phase H2 to spatially-distinct catalytic and absorptive functions. Measurement of dimensionless Péclet (Pe = convection rate/dispersion rate ~ 1.3) and Damköhler (Da = reaction rate/convection rate ~ 0.2) numbers by H2-pulse tracer experiments and kinetic studies, respectively, confirm presence of significant dispersive transport under reaction conditions and motivate development of a reaction-transport model to quantitatively examine the effects of catalyst-absorbent proximity through consideration of kinetic, diffusive, and convective length scales.
Simulation of zero-parameter reaction-transport models demonstrate introduction of zirconium metal rapidly consumes H2 via absorptive metal hydride formation, generating large axial H2 partial pressure gradients which are maintained by dispersive H2 transport. The synergy between ZrHx formation and H2 dispersion suppresses thermodynamic limits to DHA catalysis and enhances net methane pyrolysis rates throughout the catalyst bed. Simulation results quantitatively predict effluent aromatic yield and H2 partial pressure for all studied reactor configurations (i.e. staged catalyst-absorbent beds and interpellet physical mixtures).