2020 Virtual AIChE Annual Meeting
(706d) Oxygen Vacancy Healing for Desorption of Stored Ammonia from a Metal/Zeolite Nanocomposite
Authors
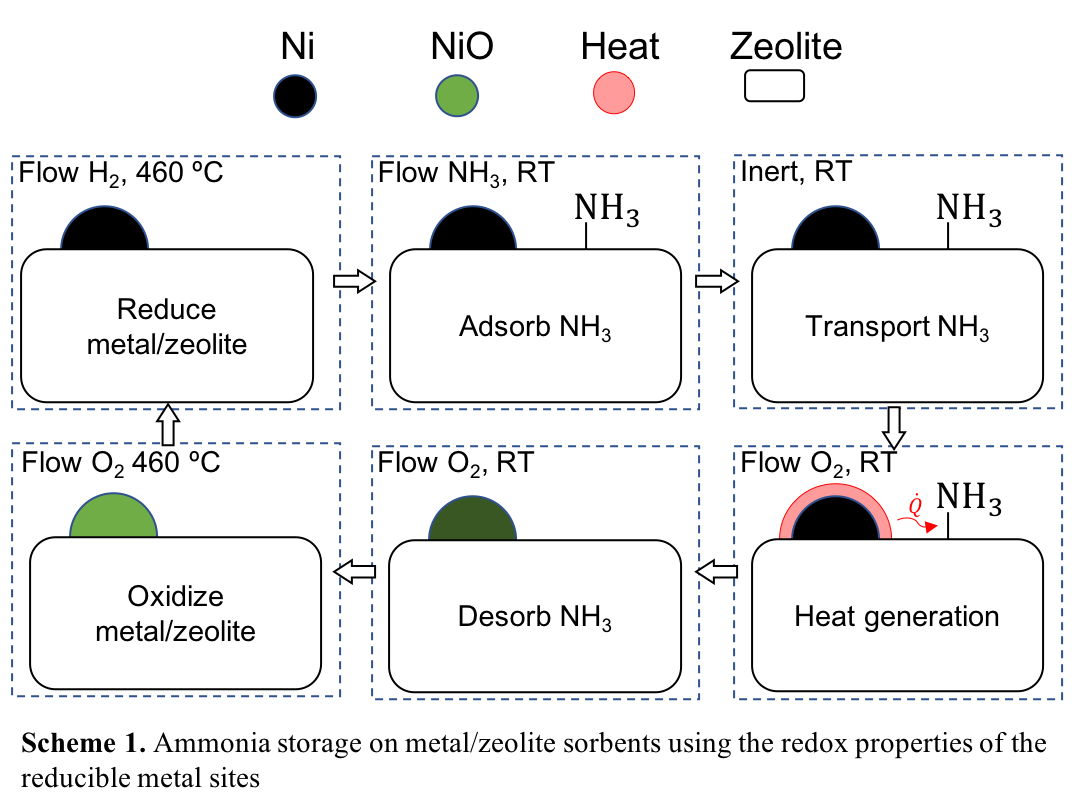
Zeolites offer highly tunable pore chemistries and geometries, which allows precise control of the chemical environment of the adsorbate. The intrinsic acidity of zeolites promotes strong gas-solid interactions but simultaneously hinders the effective release of the stored gas due to chemisorption. To overcome this strong binding energy, temperature or pressure swing is typically employed to remove adsorbed ammonia.
In the current work, the partial reduction of a nickel-oxide/zeolite nanocomposite led to a remarkable heat release upon exposure to air, which was attributed to the exothermic healing of oxygen vacancies in the NiO1-x lattice. This heating phenomenon was shown to be repeatable over 10 at least cycles. Temperatures as high at 694 ºC were measured in the catalyst bed on exposure to air. By applying the redox properties of the nickel-oxide/zeolite nanocomposite, significant desorption of ammonia was promoted. The removal efficiency was found to be 38%, an improvement in comparison to vacuum swing desorption (30%). Future outlooks are proposed to improve the total ammonia uptake and to improve the cycling process.