2020 Virtual AIChE Annual Meeting
(6f) Biosynthesis of Alkyne, Alkene, and Halogenated Amino Acids for Expanded Genetic Codes
Authors
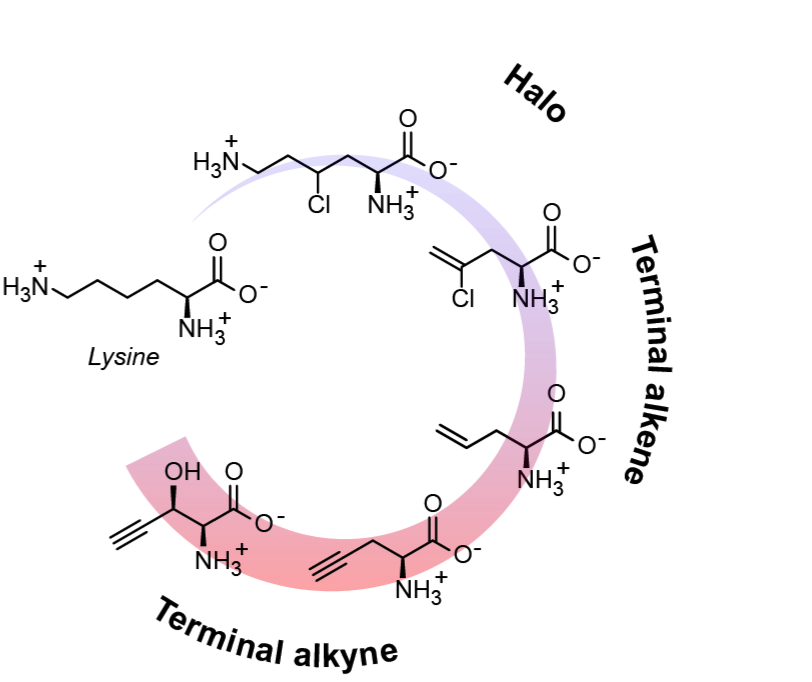
In this talk, I present the discovery and characterization of the natural, biosynthetic pathway for a terminal alkyne amino acid, β-ethynylserine (βes), from Streptomyces cattleya. Comparative genomics and comparative metabolomics were used to identify the genomic cluster and pathway intermediates, while analytical techniques were used to characterize the structure of individual chemical species. The enzymes uncovered in this pathway allow for the production of various halo, terminal alkene, and terminal alkyne L-amino acids from basic carbon sources, that can be genetically encoded and leveraged for novel applications. For example, we envision using elements of this pathway as proteomic tags that can be spatially and temporally targeted in living, complex hosts. Additionally, the βes pathway opens up the possibility of engineering industrial hosts that can seamlessly integrate halo, alkene, and alkyne amino acids into biomolecules, such as enzymes and natural product therapeutics, at scale. Taken together, this work presents an effort to bridge together advances in chemical biology (bioorthogonal reactions and genetic code expansion) with synthetic biology approaches of host engineering.
Marchand, J. A., Neugebauer, M. E., Ing, M. C., Lin, C.-I., Pelton, J. & Chang, M. C. Y. Discovery of a pathway for terminal-alkyne amino acid biosynthesis. Nature. (2019) doi:10.1038/s41586-019-1020-y.
Neugebauer, M. E., Sumida, K. H., Pelton, J. G., Mcmurry, J. L., Marchand, J. A. & Chang, M. C. Y. (2019). A family of radical halogenases for the engineering of amino-acid-based products Monica. Nat. Chem. Biol. (2019). doi: 0.1038/s41589-019-0355-x