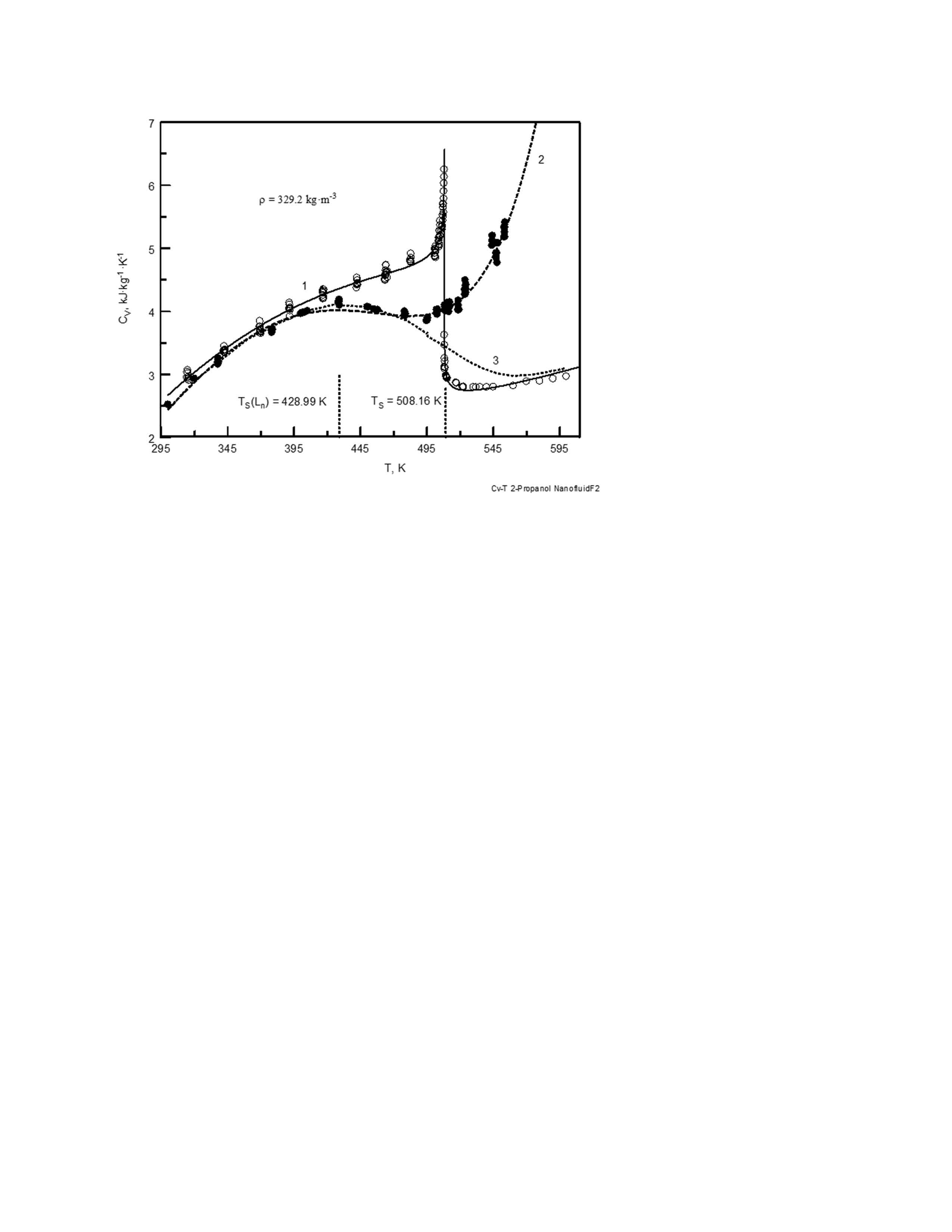
Thermodynamic properties (isochoric heat capacity, critical and phase transition temperatures, pressure and thermal-pressure coefficient) of a nanofluid (2-propanol + TiO2) were experimentally studied [1] near the critical point of the pure base fluid (2-propanol) [2] Measurements were made using a high-temperature, high-pressure, nearly constant-volume adiabatic piezo-calorimeter that was designed for simultaneous measurements of caloric (
CVVT) and volumetric (
pVT) properties of fluids along the near-critical isochores as a function of temperature in the two-phase and single-phase regions. The standard uncertainty of the density, temperature, pressure, thermal-pressure coefficient, and heat capacity measurements is estimated to be 0.1%, 0.02 K, 0.5%, 1.0%, and 1.5%, respectively. The measurements were made along a selected near-critical isochore of 282.68 kg·m
-3 for a concentration of 0.132 mass fraction of TiO2 in the temperature range from (314 to 511) K. The measured
CV and critical temperature data were interpreted in terms of finite-size scaling theory of critical phenomena for fluids confined in the finite-size media. The influence of finite size on the thermodynamic properties of the near- and supercritical fluid was studied, along with thermal instability of the nanofluid (2-propanol + TiO2) at high temperatures (near 500 K).
[1] Polikhronidi, N. G., Batyrova, R. G., Magee, J. W. and Abdulagatov I. M., "Influence of Nanofluid Instability on Thermodynamic Properties Near the Critical Point," J. Chem. Thermodyn. 133, 46-59 (2019) [DOI: 10.1016/j.jct.2018.12.009].
[2] Polikhronidi, N. G., Batyrova, R. G., Magee, J. W. and Abdulagatov I. M., "One- and Two-Phase Isochoric Heat Capacities and Saturated Densities of 2-Propanol in the Critical and Supercritical Regions," J. Chem. Thermodyn. 135: 155-174 (2019) [DOI: 10.1016/j.jct.2019.03.023].