2020 Virtual AIChE Annual Meeting
(653h) Temporal Analysis of Products Study of Ethanol Dehydrogenation As a Decisive Reaction Step for Iso-Butanol Production
Authors
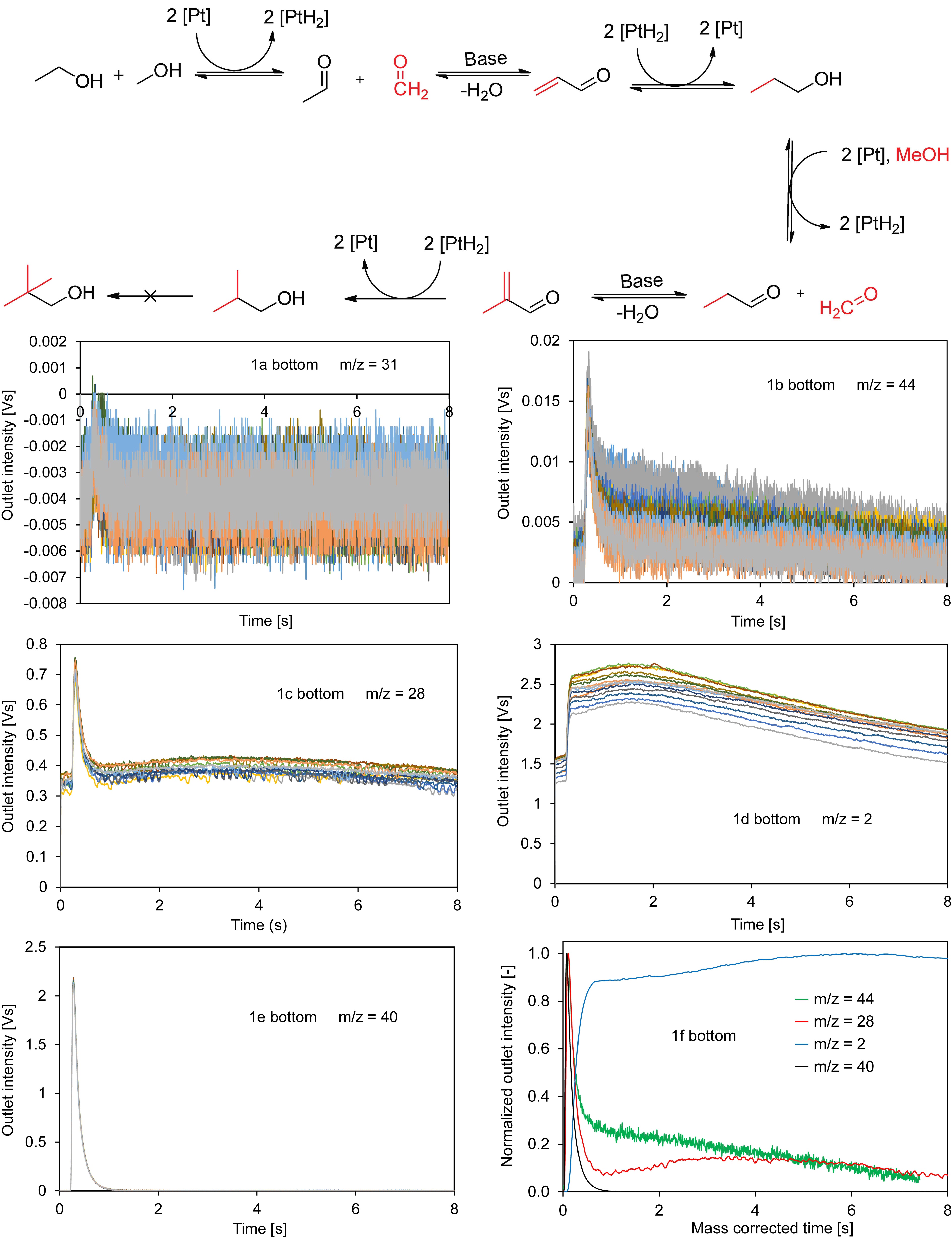
One possible route for the synthesis of iso-butanol from methanol is presented in the top part of Figure 1. It proceeds via the so called hydrogen-borrowing mechanism suggested by Siddiki et al. [1]. In this reaction scheme, the dehydrogenation of ethanol to acetaldehyde is an important step. To get a better understanding of the reaction scheme on the catalyst surface and identify possible short-lived reaction intermediates, a Temporal Analysis of Products (TAP) study of ethanol dehydrogenation on different heterogeneous catalysts has been performed, which is subject of this presentation.
In a first series of experiments, a mixture of Ar and ethanol was pulsed at temperatures between 100 °C and 200 °C over a Pt/C catalyst, which was placed in the micro-reactor of a TAP-KPC reactor system (Mithra Technologies, Inc.). The catalyst was pre-reduced in H2 at 400 °C. In this respect, Figure 1 shows in its bottom part exemplarily the outlet intensities as a function of time at a reaction temperature of 200 °C recorded for m/z values of 31 (ethanol), 44 (acetaldehyde), 28 (CO or ethene), 2 (hydrogen) and 40 (argon as the diffusion-only standard). This contribution discusses and explains these and additional experimental results and draws conclusions with respect to the reaction scheme of ethanol dehydrogenation and corresponding reaction intermediates.
[1] S.M.A.H. Siddiki, A.S. Touchy, M.A.R. Jamil, T. Toyao, K.-i. Shimizu, C-Methylation of Alcohols, Ketones, and Indoles with Methanol Using Heterogeneous Platinum Catalysts, ACS Catalysis 8 (2018) 3091-3103.