2020 Virtual AIChE Annual Meeting
(653b) Elucidating the Influence of Electric Fields Toward CO2 Activation on YSZ(111)
Authors
Nisa Ulumuddin - Presenter, Washington State University
Jung-Il Yang, Korea Institute of Energy Research
Jean-Sabin McEwen, Washington State University
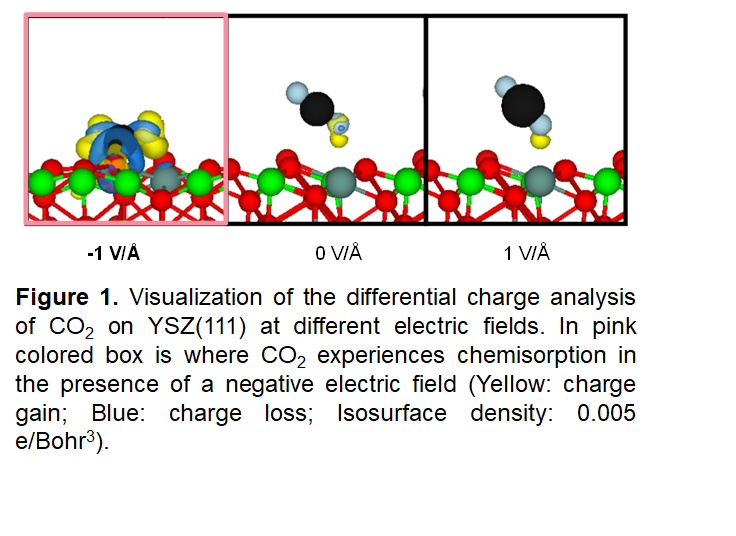
Despite its high thermodynamic stability, the presence of a negative electric field is known to facilitate the activation of CO2 through electrostatic effects.1 To utilize electric fields for a reverse water gas shift reaction, it is critical to elucidate the role of an electric field on a catalyst surface toward activating a CO2 molecule. A first-principles study was conducted to gain an atomic and electronic description of CO2 when adsorbed upon YSZ (111) surfaces when external electric fields of +1V/Å, 0 V/Å, and -1 V/Å are applied. We have found that the application of an external electric field generally causes destabilization of the oxide bonds, displaying higher reducibility. Surface reducibility was the highest when positive charges accumulate on the surface, i.e. when a positive electric field is applied. Interestingly, our findings also show that the presence of a positive field or in the absence of an electric field leads to the physisorption of CO2 with a preferential coordination to the surface Y atom rather than to Zr. In contrast, CO2 becomes chemisorbed in the presence of a negative electric field and bonds with surface O. In addition, a negative electric field leads to an accumulation of negative charge that bends CO2, altering the shape and energy of its molecular orbitals, making C more electrophilic and enabling the formation of the CO3- complex.2 Bond weakening was evident by the lengthening of the molecular C-O bonds and was investigated further in a vibrational mode analysis. The catalyst surface thus facilitates CO2 activation mainly through a charge transfer pathway. A fundamental understanding of how CO2 interacts with a field-induced surface allows us to fine-tune the environment suitable for facilitating CO2 cleavage to make value-added products.
References
- J. Phys. Chem. Lett. 1, 3256â3260 (2010).
- ChemPhysChem 18, 3135â3141 (2017).