2020 Virtual AIChE Annual Meeting
(643c) Novel Catalytic Chemical Looping Process Technology for Utilization of CO2 for Acrylonitrile Production
Authors
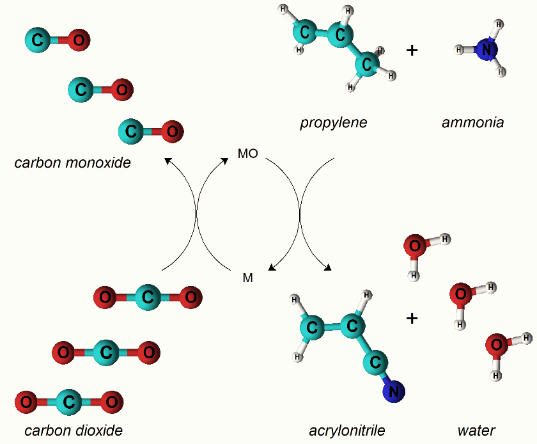
X-ray fluorescence (XRF), powder x-ray diffraction (XRD) and Tunneling electron microscopy (TEM) were used to characterize the synthesized catalysts for chemical compositions and crystallinity. Surface area was characterized by N2-physisorption using the Brunauer-Emmett-Teller theory (BET). For best performing catalysts, oxidation of the reduced catalyst by CO2 was characterized by CO2 pulsed chemisorption. CO2 to CO conversion was found to be 20-30% at 400-500°C and increased to 30-50% at 500-600°C. ACN yields ranging from 6-15% were achieved with ~ 20-40% conversion of propylene and ~ 20-25% of NH3 conversion. Selectivity for ACN was observed to be 30-40% at 500-600°C. Acrolein was observed as a by-product in all 3 cycles, confirming chemical looping of oxygen from CO2. ACN is produced primarily in the first cycle. This may be due to carbon deposition on the catalyst surface or loss of Lewis acidic sites, preventing NH3 binding, selective oxidation, or insertion of nitrogen in the ammoxidation mechanism. Future work will focus on increasing the ACN yield from all the 3 cycles.