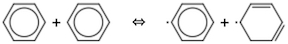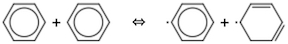Biphenyl or diphenyl is useful as a stable, high-temperature liquid because of its high boiling point (491°F or 255°C), resistant to thermal decomposition because all of its internal bonds are strong. It is produced from benzene pyrolytically, by oxidative dehydrogenation, and as a byproduct of toluene hydrodealkylation. Combination of phenyl radicals seems like an an easy explanation, with phenyls produced by O
2 or other initiating abstractors. However, straight pyrolysis has been a puzzle because of the difficulty of making phenyl from benzene, while selectivity for biphenyl is an issue for all the methods. The RMG code of Green and co-workers provides a useful means of probing chemical systems to consider a wide range of possible reactions. In the present case, it suggests reverse disproportionation of benzene to phenyl and cyclohexadienyl, leading to multiple side reactions. Application to oxidative dehydrogenation points toward optimal levels of O
2, as well. The use of RMG for probing chemical systems, aided by computational quantum chemistry, will be discussed.