2020 Virtual AIChE Annual Meeting
(598g) The Counterintuitive Dependency of Bifunctional Effect on the Degree of Ordering in Alloy Electrocatalysts for Methanol Oxidation Reaction
Authors
Tânia M. Benedetti, University of Wollongong
Jiaxin Lian, UNSW
Cameron H.W. Kelly, University of New South Wales
Robert W.J. Scott, University of Saskatchewan
Soshan Cheong, University of New South Wales
Christopher E. Marjo, University of New South Wales
Richard D. Tilley, University of New South Wales
J. Justin Gooding, University of New South Wales
Kazeem O. Sulaiman, University of Saskatchewan
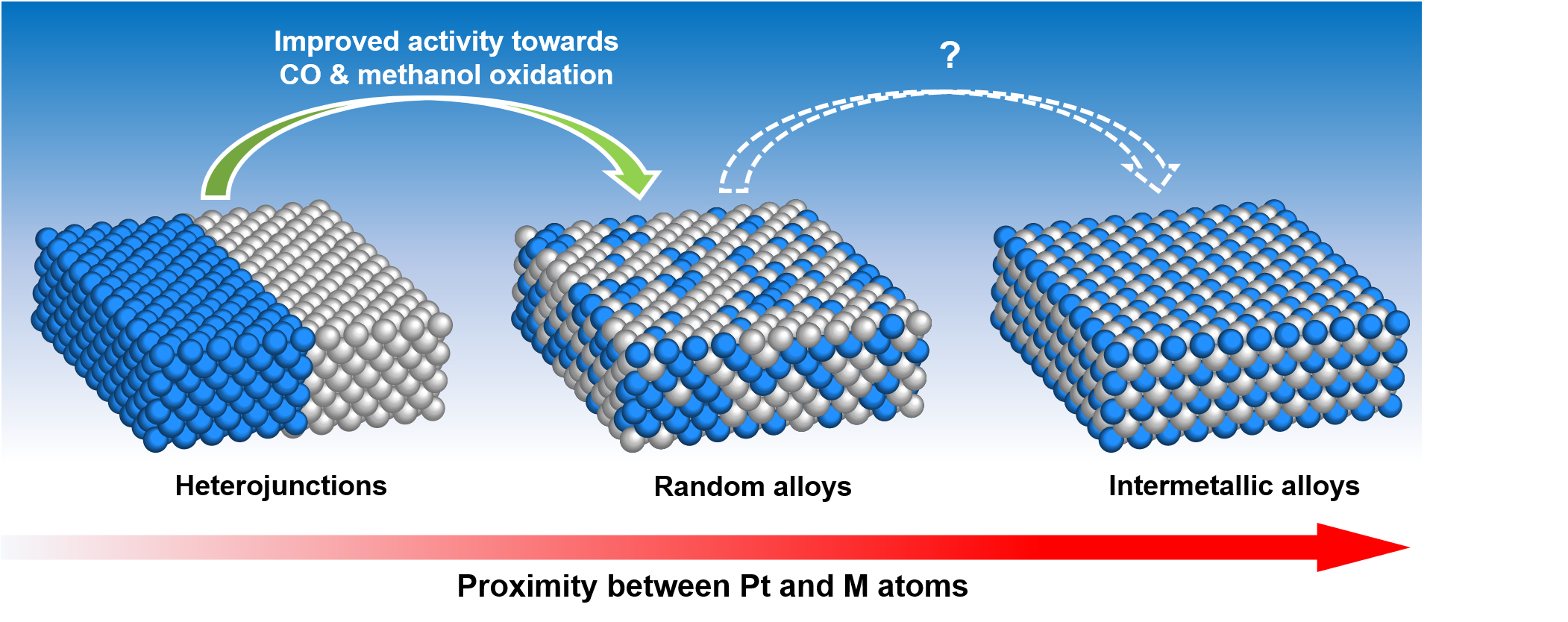
Bifunctional effect is commonly regarded as the reason why bimetallic catalysts outperform pure-metal catalysts in processes that include CO oxidation. It has been shown that alloy catalysts shorten the distance between sites for COad and sites for OHad into sub-nano range and are able to oxidize CO at much lower overpotential than non-alloyed catalysts with similar compositions. To see whether the highest intimacy between Pt and secondary metal in the ordered alloy could provide the highest activity, we modify the degree of ordering of Pt3Sn nanocubes (NCs) while maintaining the exposed {100} facets, and test them in methanol oxidation reaction and CO oxidation. Strikingly, the 60% ordered Pt3Sn NCs outperform the 95% ordered Pt3Sn NCs in methanol oxidation by almost 5 times in converted charge density. 60% ordered Pt3Sn NCs also oxidize CO at lower potential in CO stripping and are more effective in bulk CO oxidation than the 95% ordered Pt3Sn NCs and Pt NCs. Moreover, we demonstrate the outstanding CO tolerance of less ordered Pt3Sn NCs comparing to 95% ordered Pt3Sn NCs by saturating the electrolyte with CO during methanol oxidation. We reveal that the Sn atoms at the surface of the 60% ordered Pt3Sn are more prone to be oxidized under oxidative potential whereas the Sn atoms at the surface of the 95% ordered Pt3Sn NCs are resistant to oxidation. We propose that the formation of SnOx in the vicinity of Pt sites create higher proximity between OHad and COad in less ordered Pt3Sn NCs, making less ordered alloys more active for methanol oxidation reactions, in which CO oxidation plays a crucial role.