2020 Virtual AIChE Annual Meeting
(574b) Partial Oxidation of Methane to Value-Added Products over Iron Oxide Nanocatalysts
Authors
Jake Heinlein - Presenter, Yale University
Euan Gillham, University of Edinburgh
Yulian He, Yale University
Lisa D. Pfefferle, Yale University
Shu Hu, Yale University
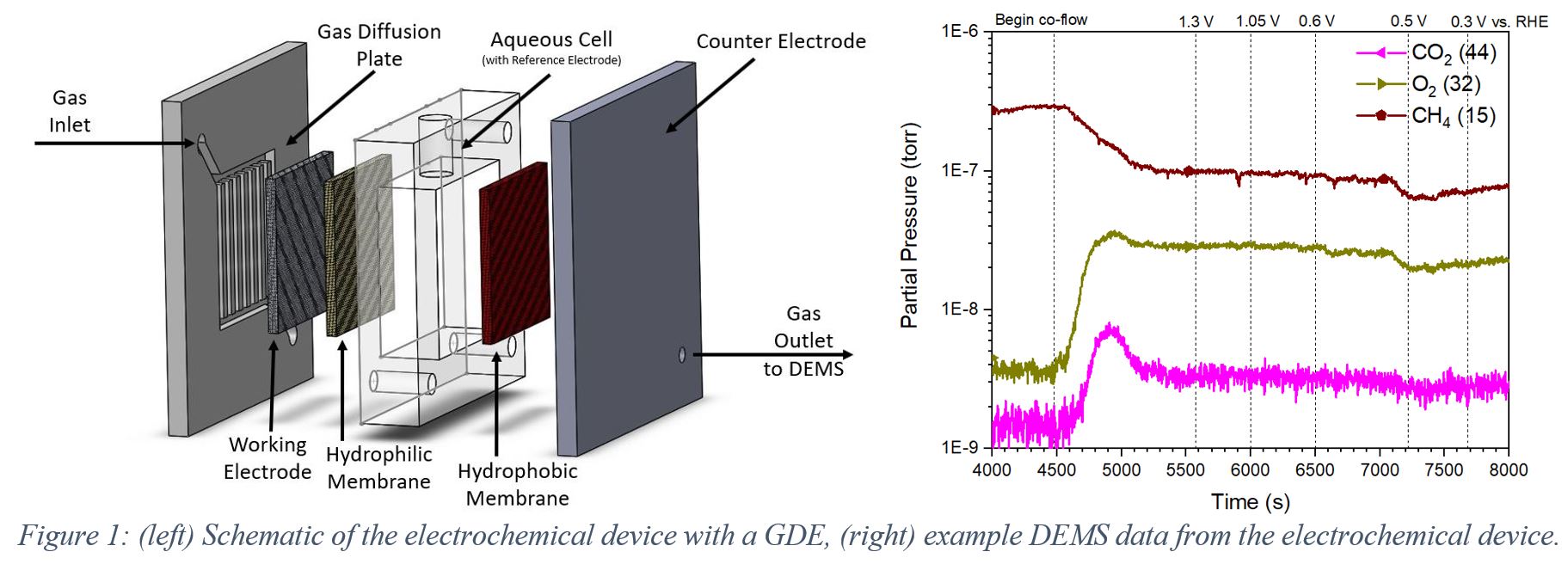
Methane, the primary component of natural gas, is a promising energy source, but billions of cubic meters are flared and wasted every year in the U.S. alone. Researchers have been working towards a process that can utilize the energy stored in methane and eliminate flaring by converting the methane on-site to a value-added product, such as methanol, for the past several decades with limited success. However, recent breakthroughs bring us closer to realizing a process fit for practical CH4 conversion. Here, we report the development of a scalable, electrochemical device that has been designed to incorporate a gas-diffusion electrode (GDE) at the interface between the methane gas and the aqueous electrolyte. The GDE is necessary to address the transport limitations of the diffusion of nonpolar methane to the electrode surface and the low solubility of methane in an aqueous electrolyte. The device is set up with an in-situ Differential Electrochemical Mass Spectrometer (DEMS) to monitor product selectivity as a function of gas composition, applied potential, and temperature. This device utilizes an earth-abundant iron oxide nanosheet catalyst with active sites that resemble the biological enzyme methane monooxygenase (MMO), which converts methane to methanol in nature. We report Faradaic efficiency and product selectivity of methane partial oxidation while co-flowing methane and oxygen. Several strategies to desorb methoxy intermediates will be discussed, which prevents overoxidation to CO2. Additionally, a basic electrolyte was chosen to sequester any produced CO2 as carbonate, which mitigates the CO2 emission. The use of a biomimetic catalyst on a GDE in our electrochemical device with in-situ DEMS has provided critical steps towards an efficient process of partial methane oxidation to value-added chemicals.