2020 Virtual AIChE Annual Meeting
(567f) Liquid Phase Membrane Supported Cyclical Flow Synthesis of Oligonucleotides for Therapeutic Applications
Authors
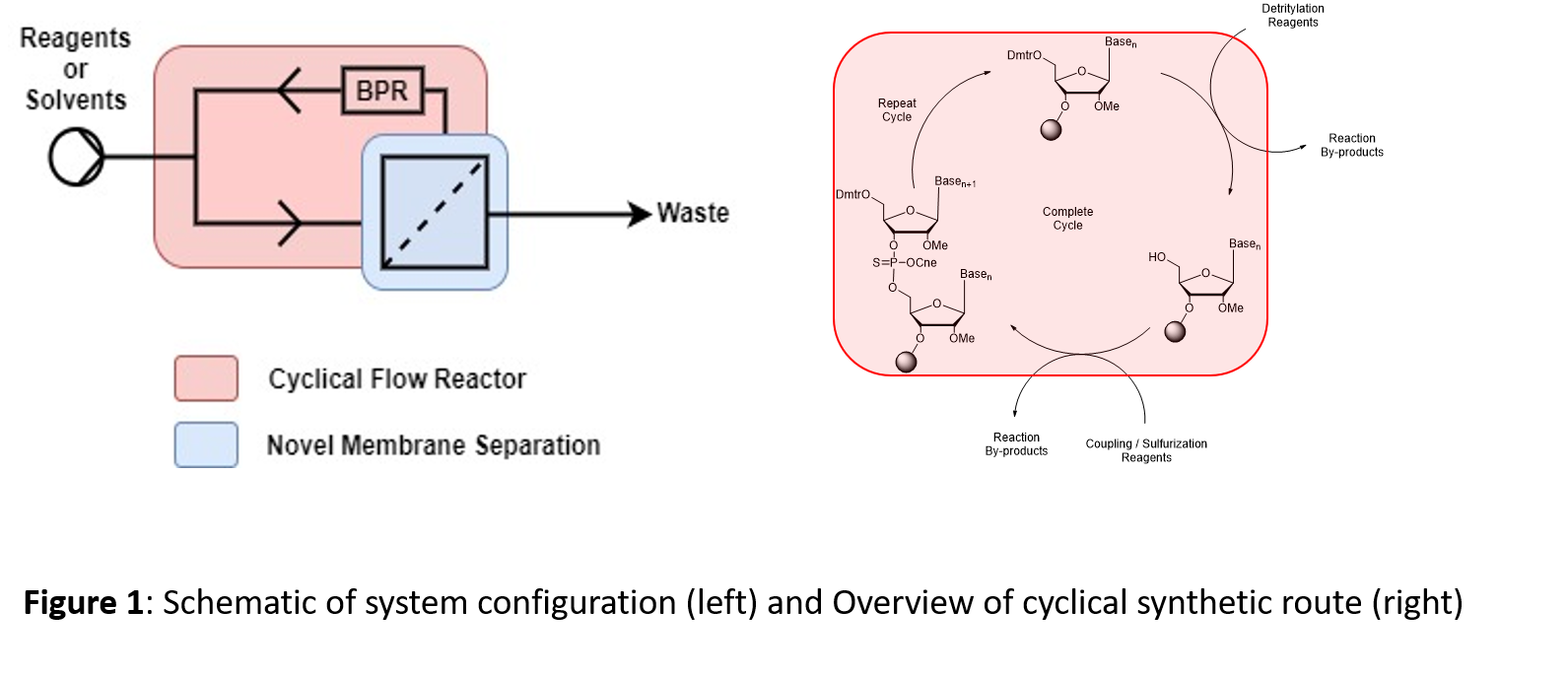
The current work demonstrates proof of concept for a novel liquid phase manufacturing process utilizing cyclical flow synthesis combined with a novel membrane separation (Figure 1). This should enable fully scalable cyclical flow synthesis of oligonucleotides with increased productivity within a compact and scalable reactor footprint. This novel process intends to meet the growing demands of the industry and promises reduced capital costs and increased product quality control.
Modifications to soluble supports were utilized to both optimize the nucleotide loading process but also to optimize membrane flux and rejection factor, and hence yield and purity with commercially available membranes. Both liquid batch and phase cyclical flow synthesis of short oligonucleotides were optimized for coupling yield, phosphoramidite consumption, solvent consumption and overall productivity. The ability to use optimum reaction/separation timescales which are inherent to coupling reactions in oligonucleotide synthesis enabled improved system performance and in turn the ability to rapidly supply oligonucleotides on demand.
Acknowledgements:
This publication is supported by Science Foundation Ireland (SFI) through SSPC, The SFI Research Centre for Pharmaceuticals (Grant number 12/RC/2275_P2).
[1] Y. S. Sanghvi, Curr. Protoc. Nucleic Acid Chem. 2011, 1â22.
[2] A. G. Molina, Y. S. Sanghvi, Curr. Protoc. Nucleic Acid Chem. 2019, 77, 1â17.
[3] A. R. Lajmi, L. Schwartz, Y. S. Sanghvi, Org. Process Res. Dev. 2004, 8, 651â657.
[4] P. R. J. Gaffney, J. F. Kim, I. B. Valtcheva, G. D. Williams, M. S. Anson, A. M. Buswell, A. G. Livingston, Chem. - A Eur. J. 2015, 21, 9535â9543.
[5] B. Burcovich, F. M. Veronese, V. Zarytova, G. M. Bonora, in Nucleosides and Nucleotides, Marcel Dekker Inc., 1998, pp. 1567â1570.
[6] M. Mutter, H. Hagenmaier, E. Bayer, Angew. Chemie 1971, 83, 883â884.
[7] F. Brandstetter, H. Schott, E. Bayer, Tetrahedron Lett. 1973, 14, 2997â3000.