2020 Virtual AIChE Annual Meeting
(547a) Understanding the Role of Small Molecules in Mineral Oxide Processing By Atomic Layer Deposited Titanium Dioxide Surfaces and Colloidal Probe Atomic Force Microscopy
Authors
Atomic layer deposition (ALD) was used to fabricate flat and smooth (RMS 0.5 nm) TiO2 surfaces. With surface charges varying from 0 to -45 mV (corresponding to pH 4.7 to pH 10.5 respectively), the interaction force between TiO2-TiO2 (colloid probe and surface) showed jump-in to contact at all pH values. When coated with SHMP, the interaction forces were purely repulsive, with no jump-in on approach or pull-off during retraction. Such behavior was consistent over a wide pH range which included the pHi.e.p. for SHMP (pH 3.4) and highly charged surfaces (pH 11).
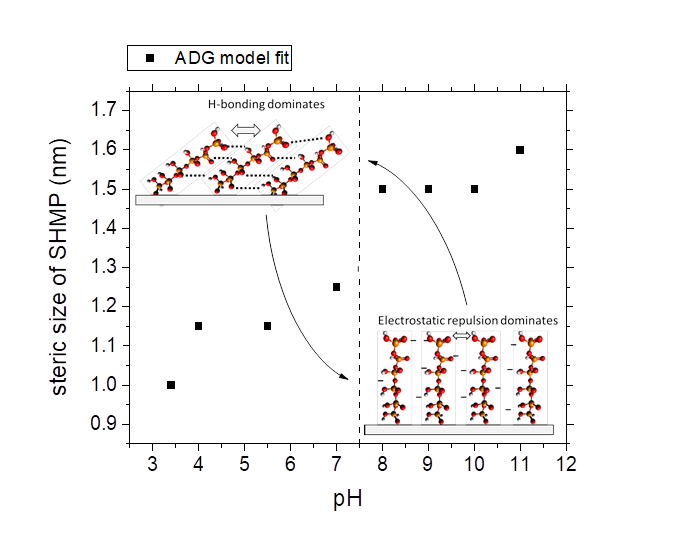
The approach data was modelled by both off-set electrical double layer forces (DLVO) and steric forces (Alexander-de Gennes model). The steric interaction was found to depend on pH with a step-change behavior in the region of pH 7.5. Below pH 7.5 the size of the SHMP molecule was ~1.0 nm and above pH 7.5 the size slightly increased to ~1.5 nm, see Fig. 1. Molecular modelling (using Avagadro software ©) estimated the length and width of SHMP to be 1.5 nm and 0.3 nm respectively. Therefore the change in steric contribution likely relates to a conformational change of the SHMP molecule at the metal oxide-liquid interface. At high pH, the hydroxyl groups of SHMP are dissociated and the molecule becomes increasingly negatively charged (confirmed by the pKa values of SHMP hydroxyl groups), with the molecule attached end-on and perpendicular to the surface. At low pH, the molecule can interact via hydrogen bonding which leads to a slight tilt in the molecule orientation.
The presentation also highlights the effect of ion-concentration (10-3 to 10-1 M), ion-type (following a Hofmeister series â CsCl, NaCl, and CaCl2), and pH on interaction forces and molecular orientation. The underlying mechanism of the small polyelectrolyte to provide excellent lubricity will be concluded.
Fig. 1. SHMP AdG modelled steric size as a function of pH (in 1 mM NaCl). Inset show the conformational change on SHMP when adopting an end-on and perpendicular orientation at high pH (dominated by electrostatic repulsion between molecules), to a slightly tilted orientation where molecules interact via hydrogen bonding when the pH is below 7.5.