2020 Virtual AIChE Annual Meeting
(528e) Kinetic Modelling and Risk Assessment of an Exothermic Fed-Batch Reaction Using Calorimetry
Authors
Calorimetry studies are an integral part of process development of synthesis steps in the pharmaceutical industry. Experimental kits, such as the Mettler-Toledo RC1 are used for this purpose with the typical aim of understanding the heat of the reaction, heat evolution, adiabatic temperature rise and therefore, safety of the chemical reactions under scrutiny. They can also see usage in reactive systems to regress kinetics of reactions, which are otherwise difficult to detect through offline composition analysis. In order to assess the thermal risks associated with a chemical reaction, both the desired and undesired (i.e., decomposition) reactions must be studied. Synthesis steps in pharmaceutical processes can be accompanied by significant heat release and must be understood well to manage them successfully at pilot and plant scales. In this study, we use mathematical modeling to predict the behavior of a reaction under different heat transfer profiles and simulate what would happen in the event of process disturbances such as a cooling jacket failure, or loss of control of fed batch (e.g. reactant added too quickly).
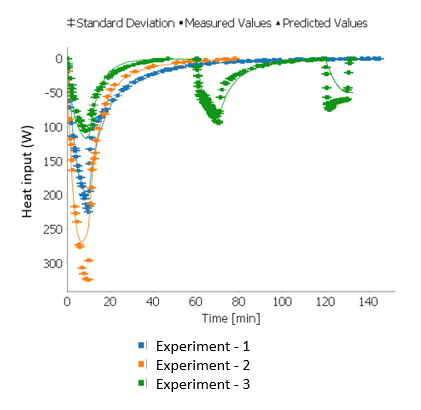
The reaction was carried out in a 1L stirred tank reactor and the reactor temperature was maintained by a cooling jacket. When the reactor temperature is well regulated, only the primary reaction occurs, however in the event of a cooling failure, an undesired decomposition reaction occurs. Experimental data from RC1 heat flow measurements were obtained using experiments executed at different process temperatures. A fed-batch reactor model was developed and simulated in gPROMS FormulatedProducts. Reaction kinetics for the primary reaction were extracted using parameter estimation by fitting the RC1 data (Figure 1).
In order to study the effect of temperature control failure, a pre-defined decomposition reaction was added to the model. Global system analysis (GSA) is an effective tool to explore design space, determine safe operating regimes for exothermic reactions, quantify effects of uncertainty, manage risk and identify opportunities for optimization. This tool was used to perform a sensitivity analysis by varying the heat transfer coefficients and initial temperatures within a specific range. The results of GSA give a clear picture of: 1) what the maximum temperature in the reactor would be if temperature control fails and hence the operation conditions that can trigger synthesis of undesirable products and impurities; 2) when the cooling failure occurs during the process, i.e. during the reactant addition or following reaction. The output from this may help to inform the control strategy for this reaction process, such as the setting of maximum reactant addition flow rates to ensure safe operation.