2020 Virtual AIChE Annual Meeting
(513n) Molecular Fractionation and Description of Food Waste Hydrothermal Bio-Crudes
Authors
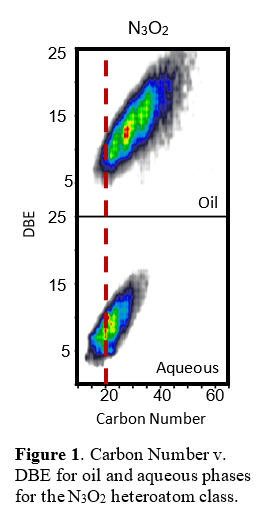
Food waste HTL remains a black box process. The reaction pathways and chemical properties of the resultant phases (gas, oil, aqueous, char) were explored using advanced analytical techniques. Oil and aqueous characterization techniques such as Fourier transform mass spectroscopy and infrared spectroscopy (FT-MS and FT-IR) were used to determine heteroatom classes present in samples. Figure 1 displays a single heteroatom class (N3O2) for oil and aqueous phases. This compound class is present in both, yet the average carbon number is much higher for the oil phase than the aqueous. The double bond equivalency (DBE) also has a greater average in the oil signifying an increased degree of aromaticity/ unsaturation in the oil. Examining the breakdown of different heteroatom classes results in the same trend, wherein both oil and aqueous phases contain the same classes with increased carbon number and DBE in the oil phase.
This provides insight into potential reaction mechanisms, leading to one pathway including the breakdown of food into water-soluble molecules before carbon polymerization reactions occur to produce heavy oil-soluble organics. We have begun to uncover the black box reaction of food waste HTL, providing deeper insight into fundamental chemical mechanisms to assist us in producing a commercially feasible process.