2020 Virtual AIChE Annual Meeting
(493d) Visible Light Mediated Switchable Selectivity in C-O, C-C and C-S Bond Formation Induced By Disulfide
Authors
Qingwei Meng - Presenter, Dalian University of Technology
Jingnan Zhao, Dalian University of Technology
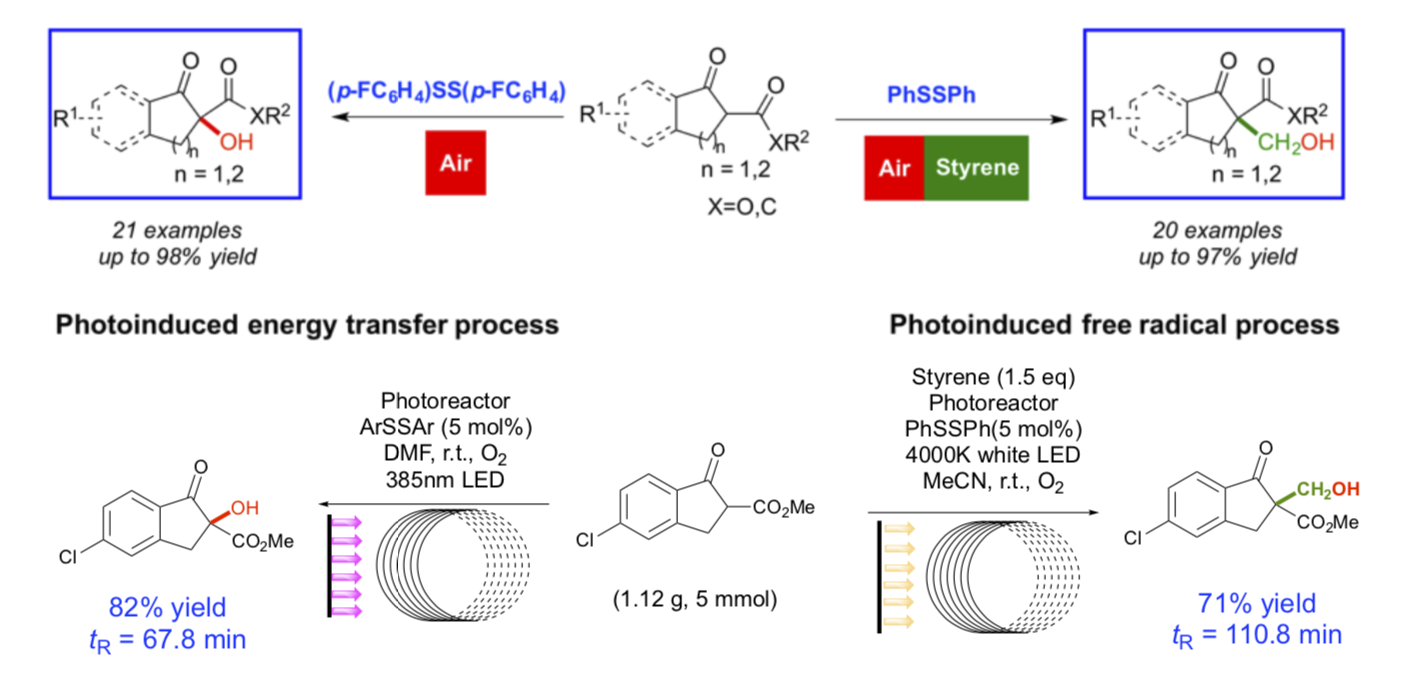
Chemists are beginning to turn their attention to the development of catalysts whose activity in a given chemical processes can be switched by an external stimulus. To achieve a mild, selectivity-controllable and general C-O, C-C and C-S bond formation, the facile selectivity-switchable functionalization of 1,3-dicarbonyl compounds represents an ideal protocol, yet a significant challenge. We thus focused on developing a bioinspired strategy. In this work, a visible light-mediated a-functionalization of 1,3- dicarbonyl compounds with switchable selectivity induced by disulfide is disclosed for the first time. Upon irradiation with visible light, the metal- and base-free α-hydroxylation and α-hydroxymethylation reactions proceeded smoothly through a disulfide-catalyzed oxidation with air under mild conditions and generated the desired products. The highly tunable selectivity between the hydroxylation (21 examples, up to 98% yield) and hydroxymethylation (20 examples, up to 97% yield) is controlled by simple changes in the stoichiometry of the styrene additive. In contrast, conducting the reaction in darkness prevented the S-S bond from being photoactivated, and the disulfides served as a sulfurization reagent and promoted the α-sulfenylation (15 examples, up to 95% yield). The reaction efficiencies of the hydroxylation and hydroxymethylation could be further improved by using a continuous-flow reactor. The combination of a continuous-flow strategy and enzyme-like switchable catalysts allowed the facile preparation of synthetically useful intermediates and products meanwhile providing a digital control and manipulation of chemical reaction paths.