2020 Virtual AIChE Annual Meeting
(464b) Development of pH-Responsible Self-Assembling Prodrug
Authors
Jin Han - Presenter, Osaka University
Keita Hayashi, Nara National College of Technology
Yukihiro Okamoto, Osaka University
Keishi Suga, Osaka University
Hiroshi Umakoshi, Osaka University
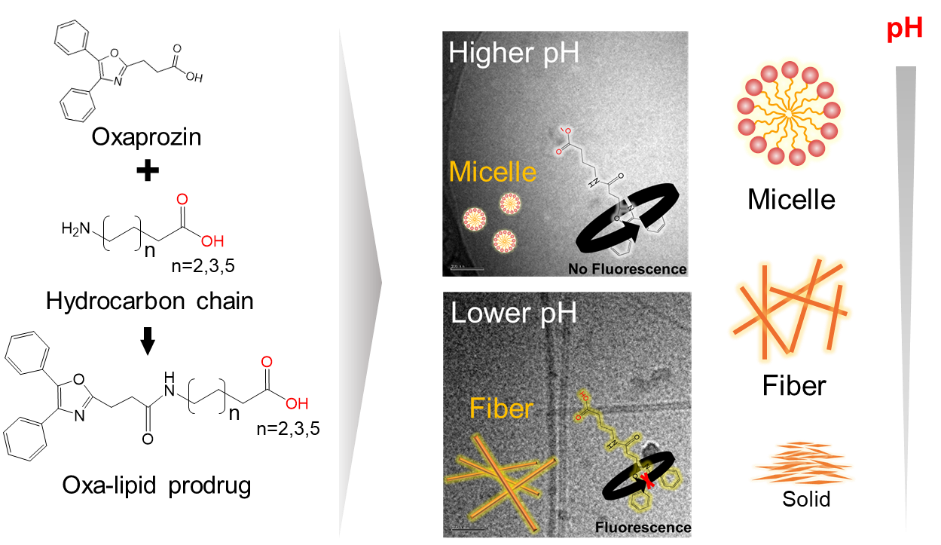
Among possible drug delivery system (DDS) strategies, the prodrug-based self-assembly DDS (SADDS) has been paid much attention in terms of high loading and controlled release. In the application of SADDS, the properties and structures of self-assembly by prodrug molecules usually play important roles in their bioactivity. However, existing strategies to prepare various structures through prodrug molecular design seem to be insufficient. In this study, we investigated pH-responsible prodrug based SADDSs as an alternate strategy, focusing on controlling self-assembly behavior via pH change and molecular structural design. The prodrug structure design was inspired by self-assembling fatty acids (FAs). Here, the aryl carboxylic acid Oxaprozin (Oxa), a primary non-steroidal anti-inflammatory agent, was selected as a model molecule to design Oxa-lipid prodrug molecules possessing carboxyl groups and different carbon chain lengths. This prodrug design strategy achieved control of the drug self-assembly behavior, changing from micelle to fiber to solid based on pH conditions and molecular concentration. Thus, our proposed study may contribute to the development of a biological environment-sensitive SADDS.