The rapid rise in global energy demand and desire for lower carbon emissions has prompted the use of renewable sources for electricity generation. With intermittent solar and wind power expected to generate 50% of all electricity by 2050 [1], energy storage solutions are needed to match energy supply with consumer demands. All-Vanadium Redox Flow Batteries (VRFBs) are a promising technology for grid energy storage because of their scalability, long lifetimes, and low maintenance. Despite these advantages, VRFBs are still limited by high costs, which can be lowered by increasing the current densities of the kinetically limiting V2+/V3+ redox couple. However, there is still a lack of fundamental understanding of the V2+/V3+ reaction mechanism, particularly the role of electrolytes in the charge transfer. In this talk, we elucidate the structure of vanadium ions in common acidic electrolytes and probe their redox kinetics at carbon electrode surfaces using Density Functional Theory (DFT) modeling and comparing with experimental measurements. We model vanadium complexes at the electrode surface to gain insight into the processes occurring at the atomic level and to help interpret experimental observations.
In our recently published work [2], we elucidate vanadium complexation with anions in common HCl and H2SO4 electrolytes by comparing experimental UV-Vis spectra to Time-Dependent Density Functional Theory (TDDFT) calculations, as well as DFT-predicted free energies of V3+-anion complexes in solution. DFT predictions using the hybrid B3LYP-D3 functional and an explicit/implicit solvent model indicate that the free energies of the complexes follow the order of [V(H2O)5SO4]+ < [V(H2O)4Cl2]+ < [V(H2O)6]3+, in agreement with our experimental observations. The V2+/V3+ kinetics are measured to be ~2.5 times faster in HCl than in H2SO4 or HCl/H2SO4, attributed to the presence of [V(H2O)4Cl2]+, where adsorbed Cl (*Cl) acts as a bridge between the carbon electrode and the vanadium ion. Previously, similar enhancement has been reported for Cr2+/Cr3+ redox couple [3], attributed to the polarizability of the bridge. A bridging mechanism through *Cl is supported by even faster redox kinetics in HBr than HCl, where adsorbed *Br being a better bridge than *Cl enhances the charge transfer.
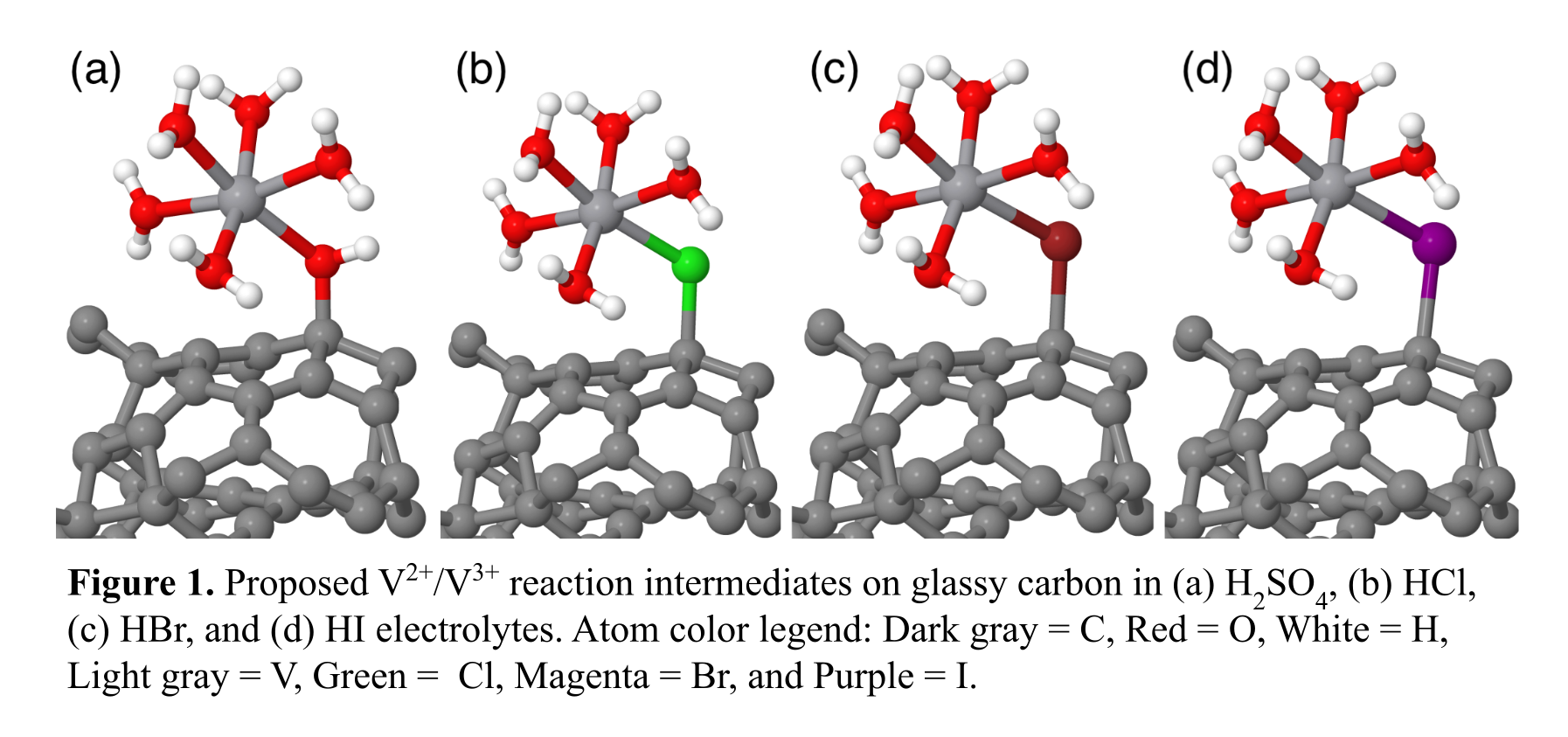
To understand the role of halide bridging ligands on the charge transfer kinetics of the V2+/V3+ reaction, we computed adsorption energies of halides (Cl, Br, and I) on a glassy carbon surface as a function of coverage using the PBE-D3 functional. These adsorption energies were used as inputs to a generalized computational hydrogen electrode model [4], where we determined that halide coverages on glassy carbon follow the trend θI > θBr > θCl at the V2+/V3+ reduction potential (E0 = -0.255 V). We also compare the energies of vanadium complexes adsorbed to a glassy carbon surface through OH, Cl, Br, and I ligands, representing the proposed reaction intermediates in H2SO4, HCl, HBr, and HI, respectively (Figure 1). The decreasing adsorption strength of [*Cl-V(H2O)5]2+ > [*Br-V(H2O)5]2+ > [*I-V(H2O)5]2+ supports our hypothesis that V2+/V3+ redox kinetics is dependent on the stability of the adsorbed intermediate. These findings open avenues in electrolyte engineering to increase V2+/V3+ redox kinetics in VRFBs.
References:
[1] Bloomberg New Energy Outlook, 2019 <https://bnef.turtl.co/story/neo2019/>
[2] Agarwal, H., Florian, J., Goldsmith, B., Singh, N. (2019). ACS Energy Letters, 2019, 4, 2368â2377
[3] Sykes, A. G. Further Advances in the Study of Mechanisms of Redox Reactions. Adv. Inorg. Chem. Radiochem. 1968, 10, 153â245.
[4] Gossenberger, F. et. al., 2015. Surface Science, 631, 17-22