2020 Virtual AIChE Annual Meeting
(423c) Membranes for Artificial Photosynthesis: A Tradeoff in Ion Transport and Product Crossover
Authors
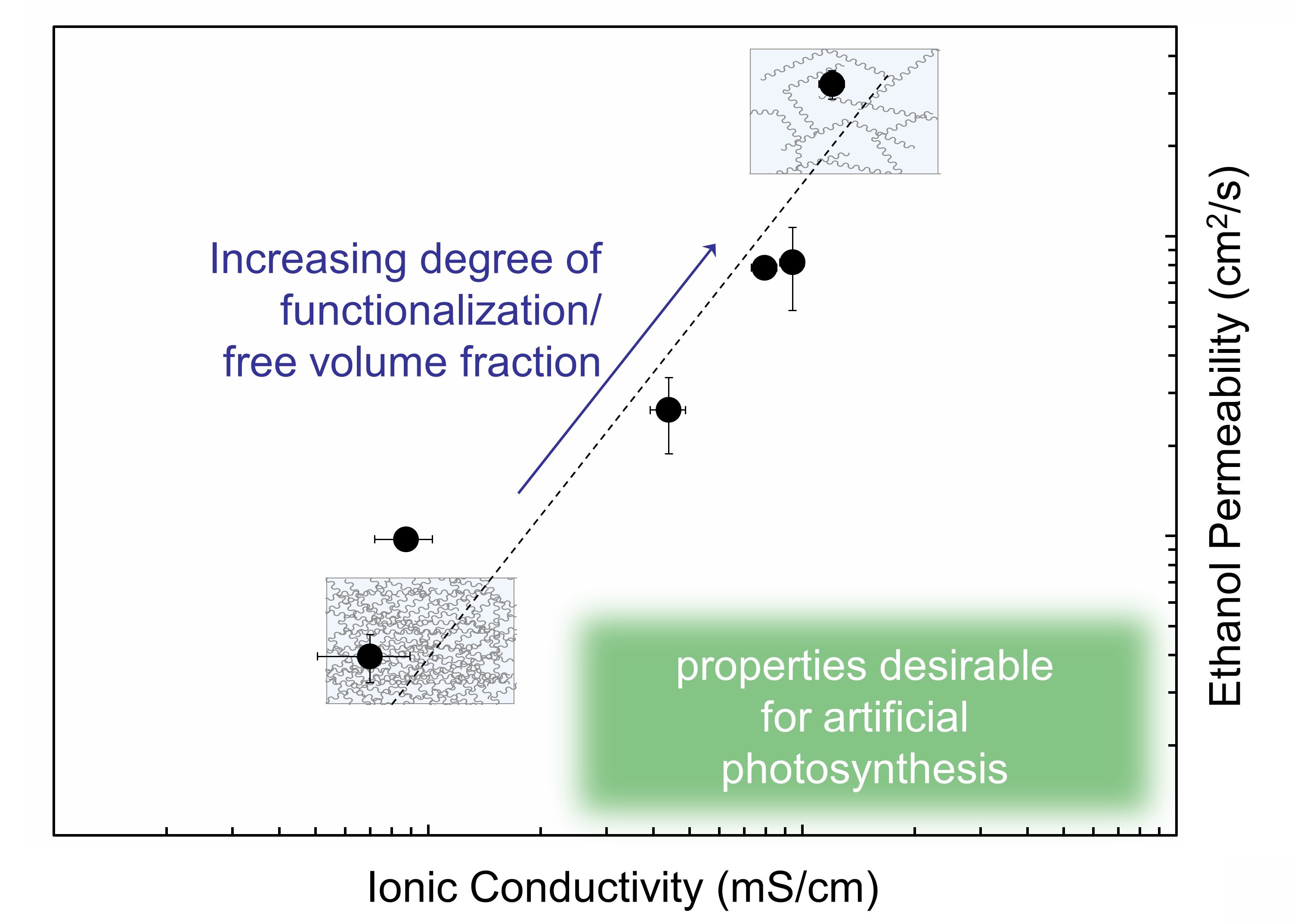
Herein, we utilize a polymer electrolyte membrane platform to explore the structure-transport relationships associated with the requirements for membranes utilized in artificial photosynthesis devices. Through systematic variation of the degree of functionalization of an imidazolium-functionalized poly(phenylene oxide) material platform, we fabricated membranes of varied water uptake and microstructure. By modifying the membraneâs microstructure, we adjusted the ethanol (a commonly reported CO2 reduction fuel product) permeation and bicarbonate (a common charge carrier) conductivity in the membranes. Membranes with a greater free volume fraction exhibited a higher bicarbonate conductivity and a higher ethanol permeability than those with a low free volume fraction. The trend of increasing ethanol permeability with increasing bicarbonate conductivity observed in this family of materials (Figure 1) presents a tradeoff in the membrane properties desirable for artificial photosynthesis (i.e., high charge carrier transport and low CO2 product crossover). While optimal membrane properties remain undefined due to the diversity of device types and operational characteristics (e.g., current density) reported, systematic control of the membrane physicochemical properties presented herein enables the study of a variety artificial photosynthesis devices operating over a range of conditions. Understanding the structure-transport relationships of the membrane could inform rational design of membranes for artificial photosynthesis and other electrochemical applications.
Figure 1. The trade-off between ionic conductivity and ethanol permeability in the imidazolium-functionalized poly(phenylene oxide) membrane platform.