2020 Virtual AIChE Annual Meeting
(327f) Activity Enhancement and Kinetics of NH3 oxidation on Pt/Al2O3:Rate Enhancement By Ball Milling and Structure Senstivity Effects
Authors
Ghosh Rajat - Presenter, University of Houston
Michael Harold - Presenter, University of Houston
Pritpal Singh Dhillon, University of Houston
Di Wang, Cummins Inc.
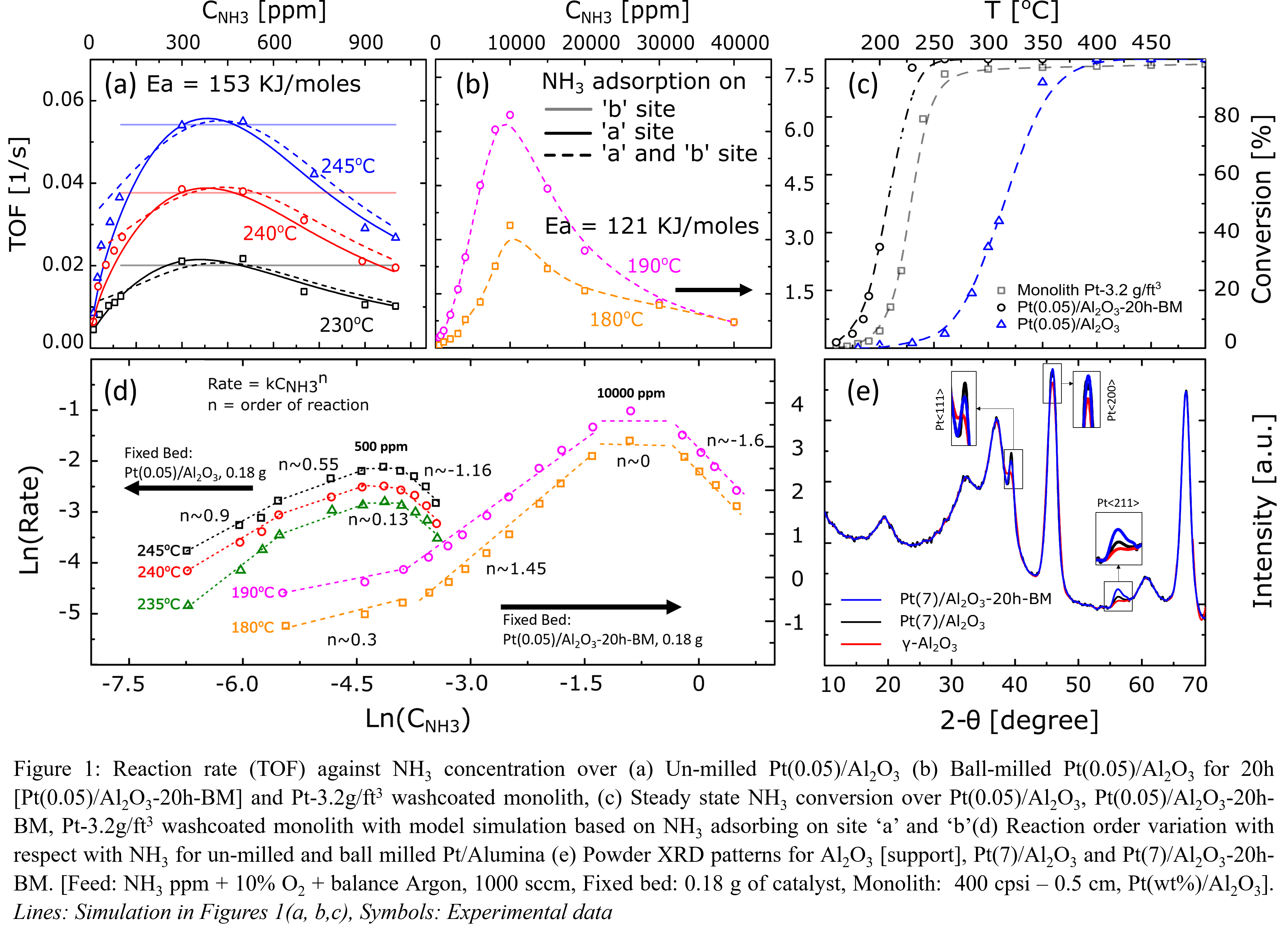
Ammonia-oxidation(AMOX) over Pt-containing catalysts is an important reaction for eliminating NH3 in diesel-engine exhaust. Kinetic models have been developed for Pt-single crystals under ultra-high vaccum[1] and for Pt-gauze for HNO3 production[2,3] but these are of limited value for NH3-oxidation on Pt-supported catalysts for emission control. Here we describe a kinetic study for Pt/Al2O3 at atmospheric pressure and the development of a microkinetic model. We measured reaction-rate(TOF) dependence over wide range of NH3 concentration from 10 â 40,000ppm for Pt/Al2O3 powder and washcoated-monoliths. The data in Figs.1(a) and (b) show the presence of rate-maximum at 500ppm for unmilled-Pt/Al2O3 but at 10,000ppm for ball-milled Pt/Al2O3 powder and washcoated-monolith. Another unexpected observation is the enhanced activity of Pt/Al2O3 samples upon ball-milling, which resulted in lowering of light-off temperature(T50) by 50°C[Fig.1(c)]. Ball-milling reduces particle size, in-turn decreases the extent of diffusion-limitations. However, steady-state AMOX done over ball-milled Al2O3 followed by Pt addition did not show the same enhanced activity ruling out diffusion-limitations as the main cause of the activity enhancement. Further characterization of Pt/Al2O3 samples using XRD[Fig.1(e)] reveals an alteration in the distribution of Pt crystalline-planes; notably, Pt<211> increased and Pt<111> decreased. These data suggest that the higher activity of ball milled-Pt/Al2O3 is a result of this crystalline-plane transformation. Microkinetics by Rebrov[1] assumed Pt<111>plane which predicted no rate variation with NH3 concentration. This is due to NH3 and O2 adsorbing on independent sites âbâ and âaâ respectively. Contrary, experiments showed â+â to â-â NH3 reaction-order variation over wide range of NH3 concentration indicates site-competition[Fig-1(d)]. The model was modified by introducing site-competition between NH3 and O2 on site-a in addition to NH3 adsorption on site-b which best described the experimental data and kinetics. These findings show the close dependence of kinetics and reactivity on Pt crystal-planes.
References:
[1]Rebrov.E.V et al.,Chem.Eng.J,90,61-76(2002)
[2]Hass.M et al.,ISCRE-25(2018)
[3]Baerns.M et al.,J.Catal,232,226-238(2005)
References:
[1]Rebrov.E.V et al.,Chem.Eng.J,90,61-76(2002)
[2]Hass.M et al.,ISCRE-25(2018)
[3]Baerns.M et al.,J.Catal,232,226-238(2005)

