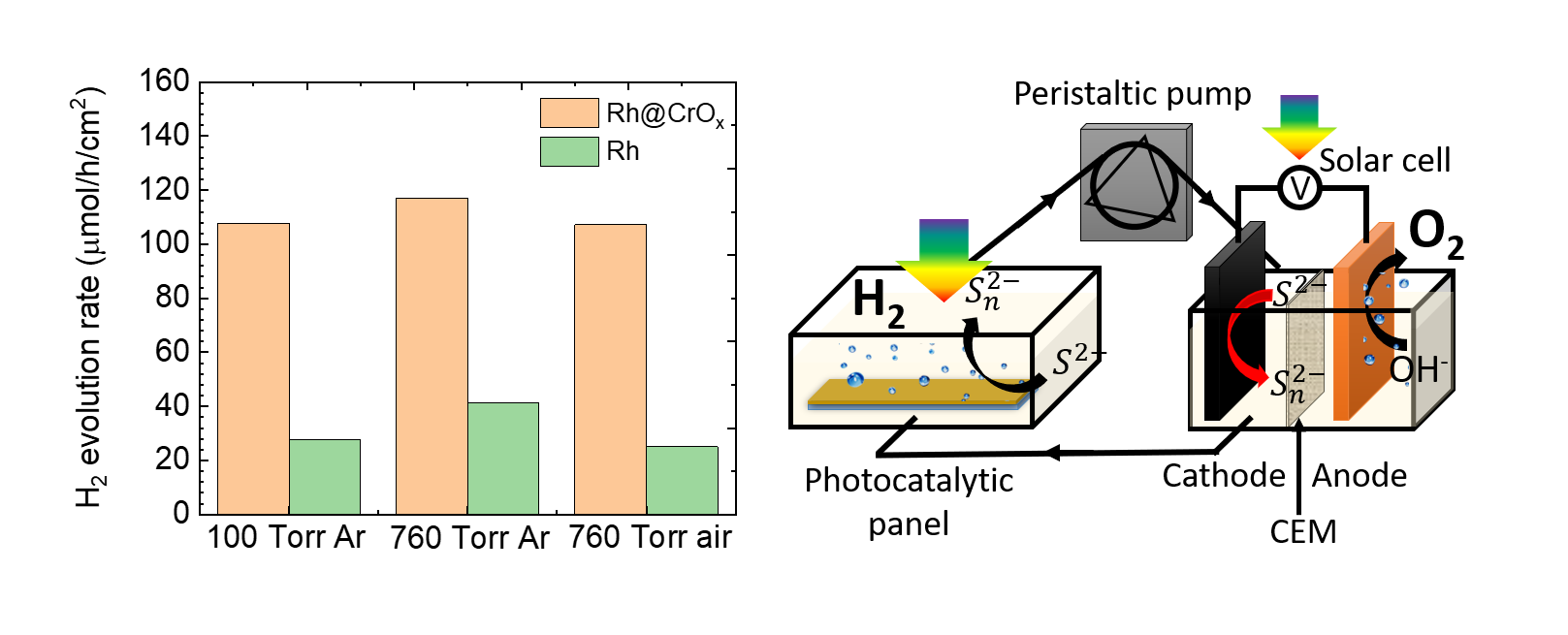
The critical step to improve the solar-to-hydrogen efficiency of particulate photocatalysis is to exploit semiconducting particles with narrow bandgaps and superior optoelectronic properties. However, group II-VI semiconductors, as a class of promising candidates, are restricted by their photo-instabilities in aqueous environment. Herein, we use a stabilization coating to address the photocorrosion of CdS photocatalysts and then construct a redox-mediated particulate solar-fuel reactor with product separation. Through the design of a highly efficient and stable CdS/TiO
2/ Rh@CrO
x photocatalytic panel, we obtain a H
2 evolution rate of 107.5 μmolâh
-1âcm
-2 under ambient condition in a Na
2S solution, which is more than 550 times higher than that of the commercial pristine CdS powders. The semiconductor/protective coating/co-catalyst interface exhibits an adaptive junction behavior, which creates asymmetric barrier height at CdS locally to facilitate lateral charge separation. We achieve water splitting by integrating a photovoltaic-electrolysis cell to regenerate the S
2-/S
n2- redox mediator and demonstrate stoichiometric H
2 and O
2 evolution with gas separation. The O
2-evolution processes are separated spatially and temporally from photocatalytic H
2 production.