2020 Virtual AIChE Annual Meeting
(233d) Ethanol Steam Reforming on SOFC System with Internal Catalyst Reforming Layer
Authors
Martinus Dewa - Presenter, The Washington State University
M. Grant Norton, Washington State University
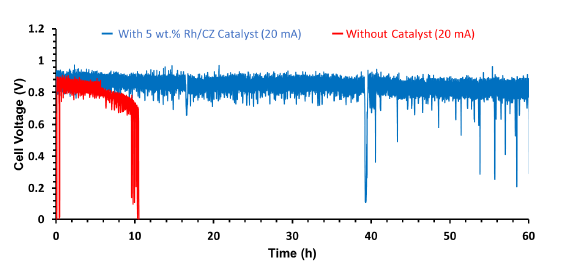
Hydrogen solid oxide fuel cell (SOFC) is a promising solution for future energy issues since it is a highly efficient and clean energy source. The commercialization of H2 SOFC is hindered by the lack of H2 infrastructure. Meanwhile, hydrocarbon fuel infrastructure is already available, but feeding hydrocarbon directly to commercial nickel-yttria-stabilized zirconia (Ni-YSZ) anode supported SOFC leads to a permanent cell deactivation due to low catalytic reforming activity and carbon deposition. This work demonstrates the effect of 5 wt.% Rh/CeZrO2 (Rh/CZ) that acts as an internal catalytic reforming layer on a direct-fed ethanol SOFC. The catalyst reforming layer was applied directly to the Ni/YSZ anode in SOFC. This additional layer will improve the reforming of the ethanol solution fuel (35 vol.%) into H2 and CO (syngas) by ethanol steam reforming reaction for 24 hours. At 800°C and under 35 vol.% of ethanol solution fuel (S/C ratio of 3), the button-typed SOFC with 5 wt/% Rh/CZ catalyst can improve the maximum current density of the cell from 150 mA/cm2 to 300 mA/cm2. The long term constant current test also shows improved cell stability from 10 h to 60 h. This additional reforming catalyst layer on SOFC can be a promising solution for future mobile SOFC applications.
Fig 01. The constant current stability plots of electrolyte-supported button cells under the direct feeding condition of ethanol (35 vol.% and the flow rate of 1.5 ml/hr) at 800â° C.