2020 Virtual AIChE Annual Meeting
(228d) Surface Engineering of Earth-Abundant Fe Catalysts for Selective Hydrodeoxygenation of Phenolics in Liquid Phase
Authors
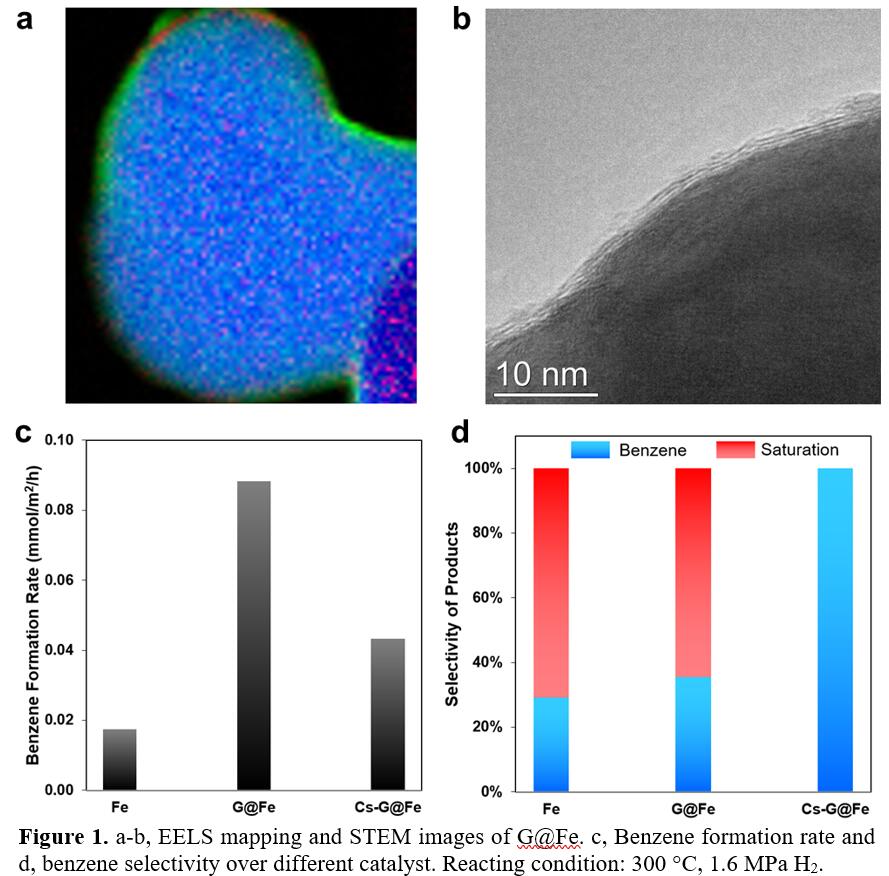
Graphene overlayers on the Fe surface can be clearly discerned by EELS elemental mapping analysis (Figure 1a), high resolution TEM (Figure 1b) and Raman spectroscopy. The graphene overlayer protects Fe substrate from oxidation. Upon addition of Cs, Fe becomes more electron rich due to electron donation from Cs to Fe through the graphene overlayers, as evidenced by XPS. The catalytic performances (i.e., phenol HDO) reveals that depositing graphene on Fe leads to a 5-fold increase of apparent reactivity compared with the bare Fe, whereas benzene selectivity remains low at ~30% (Figure 1c-d). Notably, further tailoring the graphene-covered Fe with Cs via electron tunneling modifies the surface electronic properties of the composite Cs-G@Fe catalysts such that the benzene selectivity reaches up to 100% though a negative impact on the benzene production rate was also observed (Figure 1c-d). Further ATR-IR and kinetic studies reveal that the high C-O bond cleavage selectivity is ascribed to the inhibited tautomerization of phenol, a pathway that is very facile toward aromatic ring saturation under liquid-phase conditions.