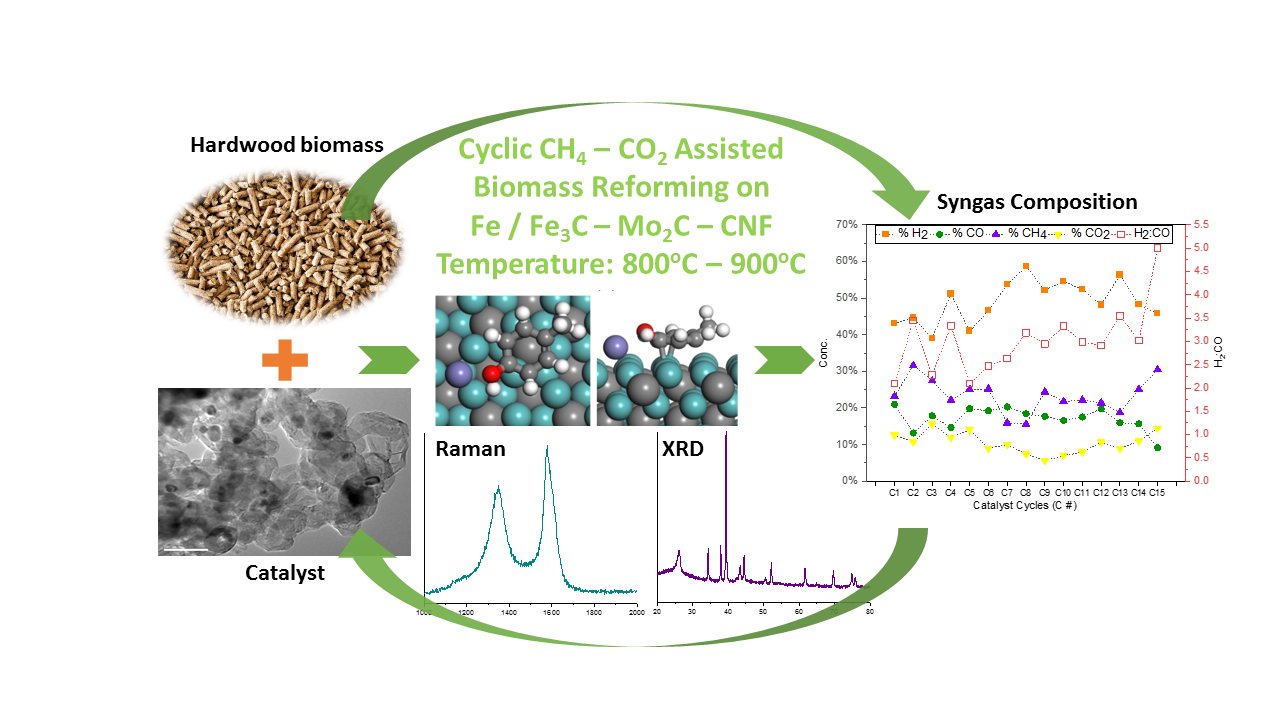
Biomass reforming for hydrogen rich syngas production was achieved through synergistic co-processing with flare gas and carbon dioxide over Fe-Mo
2C catalyst supported on carbon nanofiber (CNF). Methane â carbon dioxide activated biomass gasification can produce syngas with H
2:CO of 2 to 4 with H
2 product concentration ranging from 40 to 70 vol.%. Hydrogen rich syngas was generated over 15 cycles on Fe-Mo
2C catalyst at temperatures of 800
oC, 850
oC, and 900
oC with varying Fe loading of 0.5 to 5 wt.% and Mo
2C concentration of 4 wt.%. CNF supported catalyst subjected to multiple cycles showed excellent resistance to deactivation and was discovered to be self-regenerable. The amount of iron loading was instrumental in the nature of support synthesized from pyrolysis at 700
oC. Results showed that 0.5Fe catalyst led to synthesis of graphene supported nanoclusters of Fe and β-Mo
2C, whereas 2.5% Fe and 5% Fe loading materialized into CNF / CNT supported nanoparticles of Fe, β-Mo
2C. Conversion and gas yield nosedived to < 20% in 2 of 15 cycles on Fe-Mo
2C-CNF catalyst. These regeneration cycles possibly indicate nucleation or saturation of graphene layer synthesis or CNF growth. Nucleation of regeneration occurred mostly in cycle 2 and continued until saturated regeneration cycle varying in occurrence from subsequent cycles. Self-regeneration was the effect of free-C on the surface and complete conversion of Fe
0 active sites to Fe
3C / Fe
3C
1.5 active sites. Once elemental Fe was converted to Fe
3C
x, free-C led to 2D graphene layer formation on 2.5% Fe and 5% Fe catalysts. On graphene supported 0.5% Fe catalysts, CNF growth was observed on Fe
3C. Mo
2C active sites were much stable and did not participate in catalyst regeneration process. Self-regeneration mechanism for the CNF supported catalyst was proposed incorporating carbon from lignin oxygenates after being hydrodeoxygenated on β-Mo
2C in the framework of original mechanism from methane reforming perspective.