2020 Virtual AIChE Annual Meeting
(187c) 3D Flow Cell Simulation Via Accelerated Buffer Chemistry
Authors
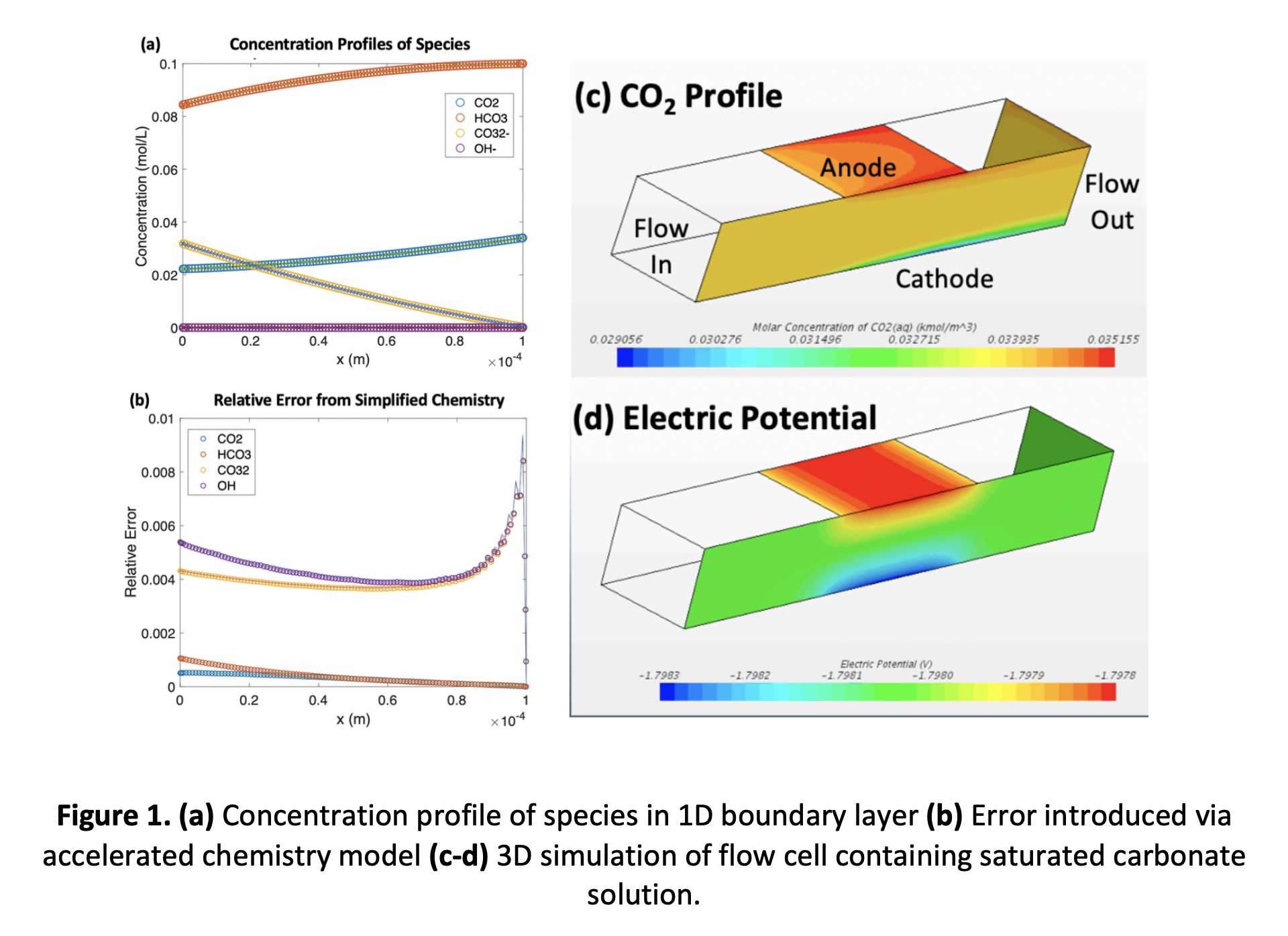
The acceleration of the bulk carbonate chemistry allows the rigorous simulation of a 3D flow reactor (Figure 1c-d) in which a CO2-saturated bicarbonate solution flows past a silver cathode at which COER and HER occur. We implement the accelerated reactive model in the commercial tool StarCCM+ and simulate the coupled fluid flow, electrostatics, and reactive mass transport in the system, including the effects of electromigration and electroneutrality. Surface kinetics are modeled via Tafel expression for COER, HER, and OER. Steady-state, 3D simulations on computational meshes with 2.65 M cells converge in ~ 5 core-hours. This simplified approach to modeling the reactor chemistry may allow the rigorous simulation of more complex 3D geometries (such as minimal surfaces or structures for convective transport enhancement) which have been shown to increase Sherwood and Nusselt numbers significantly in the heat-exchanger and membrane-reactor literature.
Prepared by LLNL under Contract DE-AC52-07NA27344. This work was supported via the laboratory directed research and development project 19-SI-005.