2020 Virtual AIChE Annual Meeting
(161ae) Broad-Spectrum Antimicrobial Polymers to Prevent the Spread of Infectious Pathogens
Authors
The first method employs the mechanism of antimicrobial photodynamic inactivation (aPDI), which relies on using a photosensitizing agent. Upon illumination with noncoherent visible light (as depicted in Figure 1), the photosensitizer is activated. In the presence of molecular oxygen, the photosensitizer generates singlet oxygen (1O2), which is cytotoxic towards pathogens and reacts non-specifically with various constituents of the cell wall in pathogens. It is highly improbable that the pathogens can develop resistance to such multimodal non-specific attack. We incorporated a photosensitizer, zinc-tetra(4-N-methylpyridyl)porphine (ZnTMPyP4+, Figure 2), capable of producing 1O2 into various polymeric substrates such as a polyethylene-based multiblock polymer, styrenic triblock copolymers, polylactic acid, and nylon-6 microfibers. In the work with the multiblock polymer,8 which is discussed below, the photosensitizer was incorporated into the polymer matrix by co-dissolution in a toluene/2-propanol co-solvent, followed by melt-pressing several times to form films. The surface distribution of the photosensitizer was determined by scanning electron microscopy and energy dispersive x-ray spectroscopy, which together revealed that, in addition to a finer distribution throughout the film, the photosensitizer occasionally formed aggregates measuring ~2 ïm in diameter. Time-of-flight secondary-ion mass spectrometry indicated a higher concentration of photosensitizer on the polymer surface relative to the bulk, consistent with blooming. The photosensitizer-containing films were tested against pathogens from the ESKAPEfamily of infectious bacteria (E. faecium, S. aureus, K. pneumoniae, A. baumannii, P. aeruginosa, and Enterobacterspecies). An antibacterial efficacy of >99.89% was achieved in all bacterial strains tested. In addition, the films were tested against two enveloped viruses (vesicular stomatitis virus, VSV, and Influenza A virus) and a non-enveloped virus (human adenovirus-5, HAd-5). The antiviral efficacy was measured as >99.96% against all the strains.
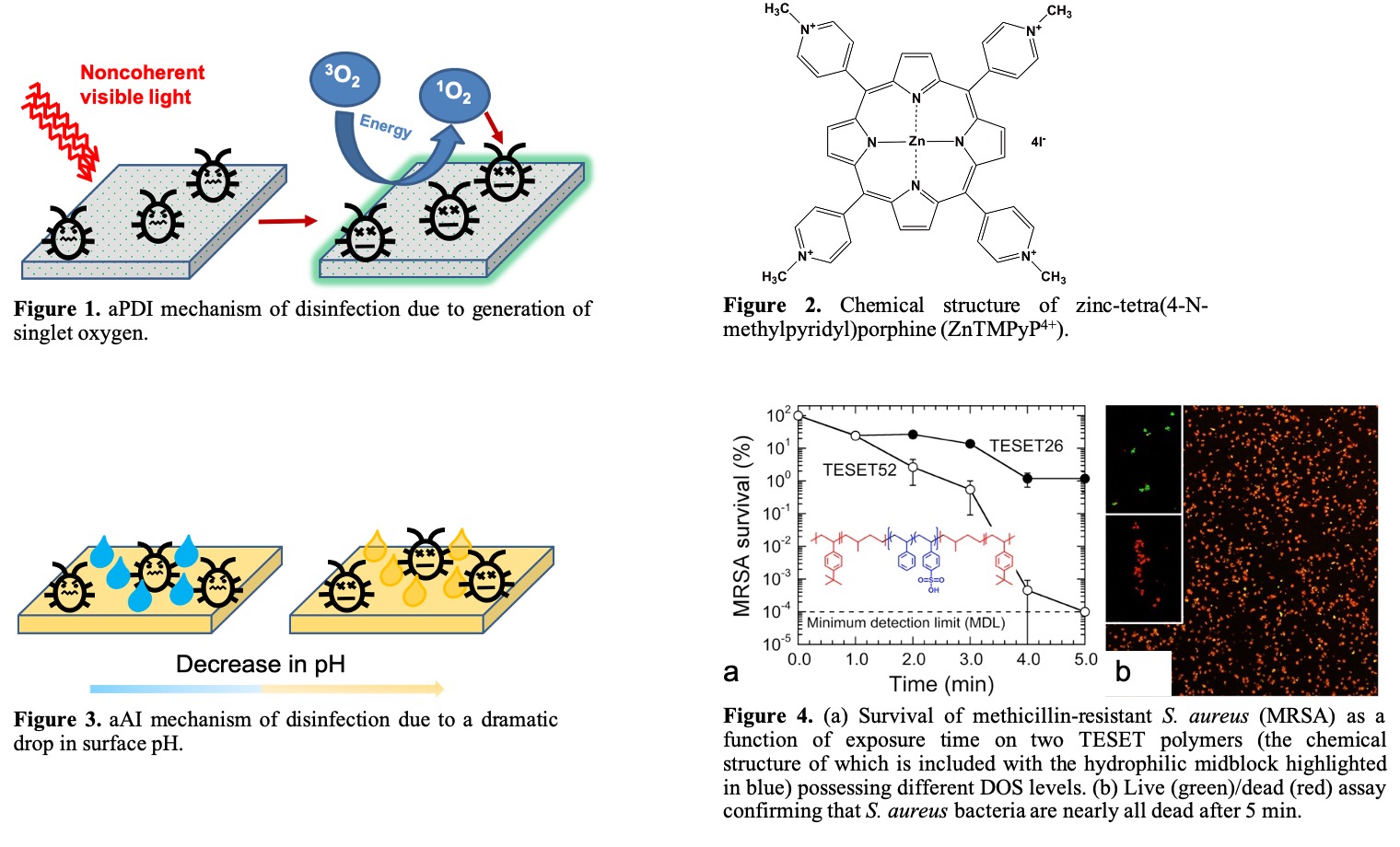
The second method involves the use of sulfonated block polymers for antimicrobial anionic inactivation (aAI). In this case, disinfection occurs by a drastic change in pH of aqueous media near the surface of the polymer (cf. Figure 3).9 An abrupt drop in pH across the pathogenic cell membrane ruptures the cell wall, as well as promotes protein denaturation and enzyme damage, which subsequently induce cell death. Several sulfonated styrenic block polymers including poly[tert-butylstyrene-b-(ethylene-co-propylene)-b-(styrene-co-styrenesulfonate)-b-(ethylene-co-propylene)-b-tert-butylstyrene] (TESETx), poly[styrene-b-(ethylene-co-butylene)-b-styrene] (SEBSx) and poly[tert-butylstyrene-b-(styrene-co-styrenesulfonate)-b-tert-butylstyrene] (TSTx) (where x is the degree of sulfonation, DOS) have been investigated. These anionic block polymers were sulfonated to various degrees (TESET: x = 26 - 52% DOS, TST: x = 17 - 63% DOS, SEBS: x =
10 - 40% DOS) and cast from tetrahydrofuran. The solvent was allowed to evaporate over 2-4 days to obtain films. These films were tested against the ESKAPE family of bacterial pathogens for exposure times ranging from 5 to 10 min. An antibacterial efficacy of 99.9999% was achieved within 5 min for all block polymers at the highest DOS (cf. Figure 4). In similar fashion as the aPDI antiviral studies, only the TESET copolymer (the chemical structure of which is included in Figure 4) was tested against enveloped viruses (VSV and Influenza A) and a non-enveloped virus (HAd-5). The enveloped viruses were eliminated to below the minimum detection limit (99.9999%), whereas HAd-5 was reduced to 99.997% with TESET52 (TESET26 was ineffective over this exposure time). A recent study10 focusing on the survivability of the SARS-CoV-2 virus on a variety of surfaces suggests that the virus can remain alive on inanimate objects for at least several hours. This observation, combined with a current lack of antivirals/vaccines, makes this virus a serious global threat, resulting in considerable worldwide proliferation and a mindboggling number of human fatalities. To tackle the ongoing tragedy of this pandemic and provide a preventative solution to thwart the spread of virus through surface contact, we have tested TESET films against human
coronavirus 229E (an alpha coronavirus strain that commonly infects humans and serves as a proxy for SARS-CoV-2) at several exposure times. We have found that, after a relatively short exposure time, TESET52 inactivates the virus to below the minimum detection limit of 10-4%, thus providing evidence for an effective new addition to the arsenal being developed to mitigate the present and possibly upcoming spread of COVID-19, a highly contagious and dangerous viral disease.
References:
- Cimolai, N. MRSA and The Environment: Implications for Comprehensive Control Measures. Eur. J. Clin. Microbiol. Infect. Dis. 2008, 27, 481.
- Antibiotic Resistance Threats in the United States, 2019. Atlanta, GA: U.S. Department of Health and Human Services, CDC; 2019. (http://www.cdc.gov/ drugresistance/Biggest-Threats.html.)
- OâNeill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; Review on Antimicrobial Resistance: London, 2016 (amr-review.org).
- Scott, R. D., II The Direct Medical Costs of Healthcare-Associated Infections in U.S. Hospitals and the Benefits of Prevention; Centers for Disease Control and Prevention: Atlanta, 2009 (cdc.gov/hai/ pdfs/hai/scott_costpaper.pdf).
- Zhang, H.; Oyanedel-Craver, V. Comparison of the Bacterial Removal Performance of Silver Nanoparticles and a Polymer Based Quaternary Amine Functionalized Silsesquioxane Coated Point-of-Use Ceramic Water Filters. J. Hazard. Mater. 2013, 260, 272.
- Santo, C. E.; Lam, E. W.; Elowsky, C. G.; Quaranta, D.; Domaille, D. W.; Chang, C. J.; Grass, G. Bacterial Killing by Dry Metallic Copper Surfaces. Appl. Environ. Microbiol. 2011, 77, 794.
- Kubacka, A.; Diez, M. S.; Rojo, D.; Bargiela, R.; Ciordia, S.; Zapico, I.; Albar, J. P.; Barbas, C.; Martins dos Santos, V. A. P.; Fernández-García, M.; Ferrer, M. Understanding the Antimicrobial Mechanism of TiO2-Based Nanocomposite Films in a Pathogenic Bacterium. Sci. Rep. 2015, 4, 4134.
- Peddinti, B. S. T.; Scholle, F.; Ghiladi, R. A.; Spontak, R. J. Photoactive Polymers as Comprehensive Anti-Infective Materials: Staying Ahead of a Growing Global Threat. ACS Appl. Mater. Interfaces 2018, 10, 25955.
- Peddinti, B. S. T.; Scholle, F.; Vargas, M. G.; Smith, S. D.; Ghiladi, R. A.; Spontak, R. J. Inherently Self-Sterilizing Charged Multiblock Polymers that Kill Drug-Resistant Microbes in Minutes. Horiz. 2019, 6, 2056.
- Van Doremalen, N.; Bushmaker, T.; Morris, D. H.; Holbrook, M. G.; Gamble, A.; Williamson, B. N.; Tamin, A.; Harcourt, J. L.; Thornburg, N. J.; Gerber, S. I.; Lloyd-Smith, J. O.; de Wit, E.; Munster, V. J. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564.