2020 Virtual AIChE Annual Meeting
(144c) Identifying the Processing Space of Continuous Granulation: A Case Study of Extended Release Tablets
Author
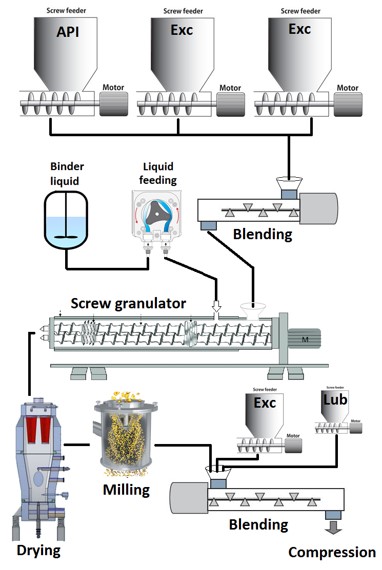
Using batch high shear granulation, we evaluated the formulation and process parameters that can produce metoprolol succinate extended release tablets. These formulation and process parameters were successfully transferred to continuous twin screw granulation to produce the same dissolution properties of the finished product. The effects of the screw configuration parameters on the characteristics of the granules were further investigated. The interaction between the processing, e.g., liquid to solid ratio, screw speed, barrel fill volume, etc., and the significant screw parameters was studies to unveil the processing space and to identify the failure modes.
Similar granule quality attributes were obtained for most of the formulations; although, the conditioned bulk density and compressibility of the granules determined the dissolution properties of the tablets. The number of kneading and sizing elements had the most significant impact on granule characteristics. The effects of the staggering angle and distance between the kneading zone of the screws were negligible.
This presentation will then highlight the processing space of screw granulation to produce extended release tablets. Some of the failure modes will be discussed along with the feasibility of implementing PAT tools to monitor the granulation process and granules properties.
DISCLAIMER
This presentation reflects the views of the presenter and should not be construed to represent FDAâs