2020 Virtual AIChE Annual Meeting
(127f) CO2 Electroreduction on Modified Cu Catalysts: Using Subsurface Dopants to Enhance Catalytic Performance
Authors
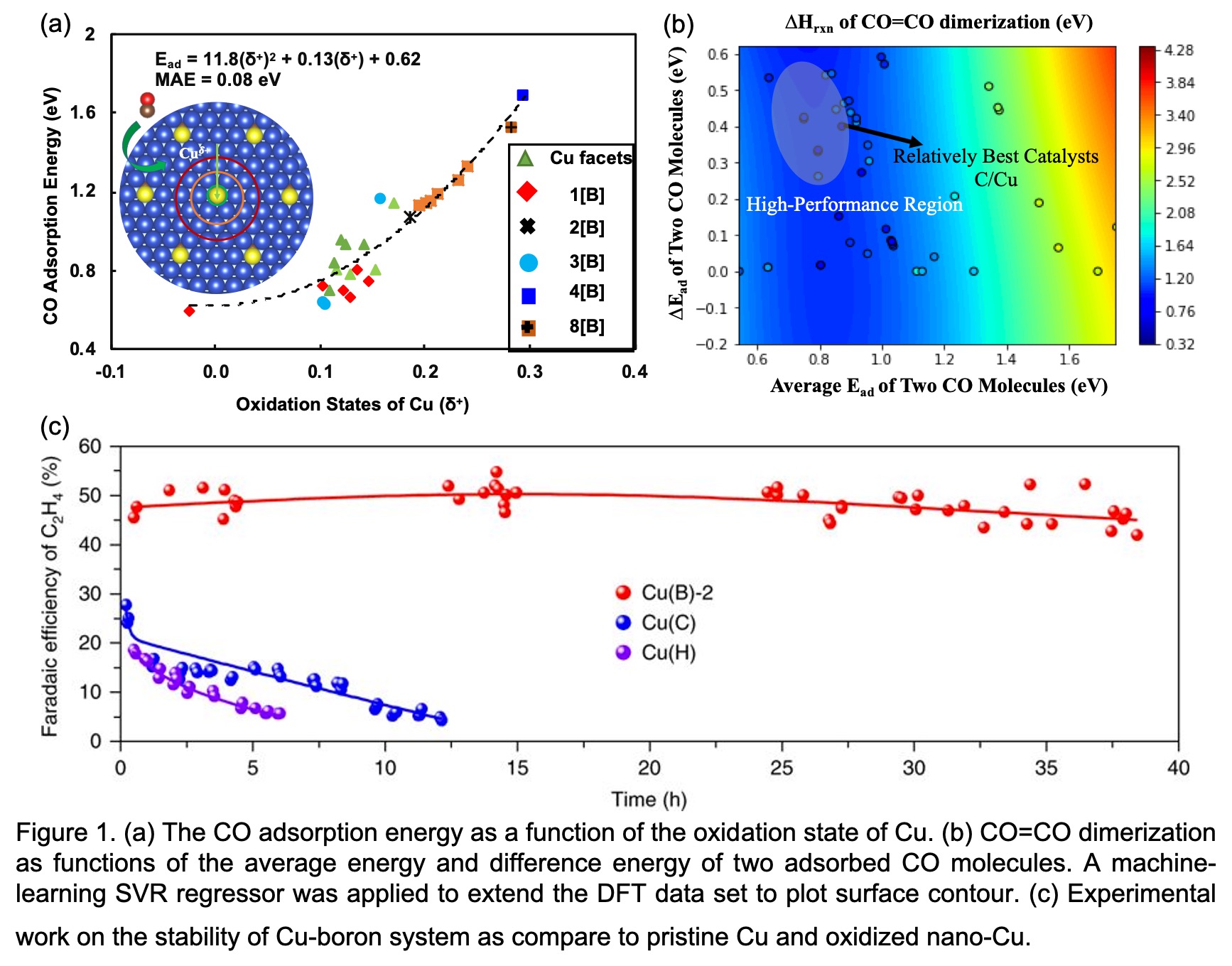
We took the view that the electronegativity of new dopants could increase Cuδ+ sites and offer a subsurface doping strategy that keeps the Cuδ+ sites stable under the reducing potentials used in CO2RR. In DFT calculations (Figure 1), we found that boron and carbon elements under negative applied potential can be stable at the subsurface of a Cu(111) slab, and that they greatly alter the Cu oxidation state to be positive, and as a consequence enhance the selectivity of ethylene products from CO2RR. Our corresponding experiments also achieve a high Faradaic efficiency for ethylene of ~52% for boron-doped Cu and ~78% for carbon-doped Cu. Importantly, boron-doped and carbon-doped copper showed ~40 h and ~170 h stability for CO2RR to ethylene. This theoretical and experimental combined work indicates a role for subsurface chemistry in enhancing the selectivity and stability of the Cu based CO2RR system.