Introduction: Hydrogels are attractive matrices for cell delivery and tissue regeneration due to their injectability, ease of cell encapsulation, and tunable chemical and physical properties. However, conventional hydrogels often lack macroporosity, which hinders vascular ingrowth, cell survival, proliferation, and tissue formation. To overcome the limitations associated with conventional hydrogels, our lab has recently reported the development of gelatin-based microribbon (μRB) hydrogels, which combines the injectability with macroporosity, and enhanced stem cell survival and bone regeneration in vivo [1]. However, one remaining bottleneck is that the rate of new bone regeneration in vivo remains slow. Bone morphogenic protein 2 (BMP2) and vascular endothelial growth factor (VEGF) are two potent growth factors with demonstrated efficacy of enhancing osteogenic differentiation of MSCs, vascularization and new bone formation in vivo [2]. To harness the benefit of drug delivery to enhance μRB scaffold-mediated bone repair, there remains a need to design drug delivery components that can be stably incorporated into macroporos μRB scaffolds while facilitating tunable release of multiple growth factors in situ. The goals of this study are: (1) to develop a polydopamine-coated, mesoporous silica nanoparticle (MSN)-based drug delivery system for controlled co-delivery of VEGF and BMP2 that can be stably incorporated into gelatin μRB-based scaffold, and (2) to validate the efficacy of drug-eluting μRB scaffolds to accelerate endogenous bone formation in vivo using a mouse critical-size cranial defect model.
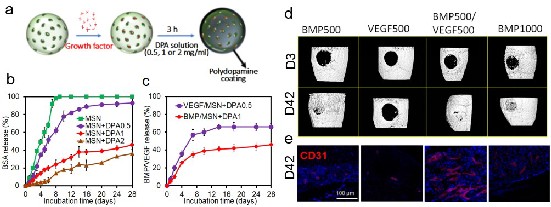
Materials and Methods: MSNs were chosen given its demonstrated biocompatibility, osteogenic potential, low-cost, and efficacy as drug delivery vehicles. Dopamine (DPA) was chosen as it is a powerful bioinspired adhesive with high binding efficiency with proteins. To tune release kinetics, MSNs were first loaded with protein (1 µg/ml) for 30 mins, then coated with varying concentration dopamine (0, 0.5, 1 and 2 mg/ml) (Fig. 1a). All groups of MSNs were then encapsulated in µRB scaffolds by photocrosslinking, incubated in PBS at 37 ºC and supernatant were collected at multiple time points for up to28 days. Bovine serum albumin was used as a model protein first to determine the effects of varying polydopamine coating on release kinetics. Optimized polydopamine coating were subsequently repeated with VEGF and BMP2, and release was measured using ELISA. Further, To evaluate the efficacy of VEGF and BMP2 co-delivery from polydopamine-coated MSNs on bone formation, 500 ng VEGF (VEGF500) and 500 ng BMP2 (BMP500) embedded within polydopamine coated-MSNs with MSN+DPA0.5 and MSN+DPA1 formulations, respectively, and encapsulated in acellular μRB scaffold (BMP500/VEGF500), were implanted in a mouse critical size cranial defect for 42 days. The outcomes were evaluated using X-ray microtomography (μCT) to assess the mineralization level of the implants as well as immunostaining of CD31-positive cells for endothelial cells to assess the extent of vascularization within the implants.
Results and Discussion: Using BSA as a model protein, we demonstrated tunable protein release kinetics from MSN by varying dopamine coating. Increasing dopamine coating concentration led to increased dopamine polymerization, which led to slower protein release due to decreased diffusion. While 100% of the protein were released from uncoated MSNs after 8 days, MSNs coated with 0.5 ,1 or 2 mg/ml of dopamine reduced the total accumulated protein release to 80%, 38% and35% of the initial loading amount by day 28, respectively (Fig. 1b). Based on the release kinetics of BSA, we chose 0.5 mg/ml and 1 mg/ml of dopamine coating for MSNs loaded with VEGF and BMP2, respectively. This allows a fast release of VEGF (>50% within the first week) to induce early vascularization, and slower BMP2 release (>50% within 3-4 weeks) to support bone regeneration. ELISA results confirmed dual release kinetics of VEGF and BMP2 from μRB scaffolds containing MSNs in vitro (Fig. 1c). When implanted into a mouse critical size cranial defect model, co-delivery of VEGF500 and BMP500 in μRB scaffold resulted in the fastest new bone formation with highest bone mineral density compared to single factor delivery alone, as shown by microCT (Fig. 1d). Co-delivery of VEGF/BMP2 (500 ng each) also led to faster and more robust bone formation than higher dose of BMP2 delivery alone (1000 ng), indicating the synergistic effect of VEGF/BMP2 on bone formation (Fig. 1d). In addition, CD31 immunostaining for endothelial cells revealed that BMP500/VEGF500 group induced the highest vessel density among all groups (Fig. 1e) .
Conclusions: Here we report macroporous gelatin µRB hydrogels with MSN-based drug delivery components for bone repair. Using BMP2 and VEGF as model proteins, we demonstrate dopamine-coated MSNs support tunable protein release with retained bioactivity in vitro and in vivo. Co-delivery of VEGF and BMP2 from polydopamine-coated MSNs in gelatin μRB scaffold significantly enhance vascularization and accelerate endogenous bone regeneration in vivo in a mouse cranial defect model. Given the modular design of MSNs, they can be easily adapted to load multiple soluble factors withindependently tunable release kinetics, thereby substantially enhance and accelerating the efficacy of µRB-based scaffold for regenerating different tissue types.
Acknowledgements: The authors would like to thank NIH R01DE024772, NIH 1R01AR074502, Stanford coulter translational grant, Stanford bio-X IIP seed grant, and Stanford SPARK program for funding.
References:
1. Han, L. H.; Yu, S.; Wang, T.; Behn, A. W.; Yang, F., Microribbon-Like Elastomers for Fabricating Macroporous and Highly
Flexible Scaffolds that Support Cell Proliferation in 3D. Advanced Functional Materials 2013, 23 (3), 346-358.
2. Carragee, E. J.; Hurwitz, E. L.; Weiner, B. K., A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. The Spine Journal 2011, 11 (6), 471-491.
Figure 1. (a) Schematic of fabricating drug-eluting MSNs without or coated with varying concentration of DPA. (b) Tunable release kinetics from MSNs with varying DPA coating concentrations using BSA as a model protein. (c) Dual release kinetics of VEGF and BMP2 from MSNs was achieved with optimized DPA coating. (d) Representative μCT images of mouse cranial defects at days 3 and 42 after treatment with μRB scaffolds containing BMP500, VEGF500, BMP500/VEGF500 or BMP1000. The results demonstrate that co-delivery of BMP2/VEGF (BMP500/VEGF500) induced the fastest bone regeneration rate after 42 days. (e) Representative staining of CD-31 (for endothelial cells) in cranial defects at day 42 in vivo (Red: CD31, Blue: DAPI). The images show that BMP500/VEGF500 resulted in highest extent of vascularization within the defect site.